OCR Specification focus:
‘Describe the nuclear atom model with protons, neutrons, and orbiting electrons.’
The simple nuclear model represents atoms as tiny nuclei containing protons and neutrons surrounded by orbiting electrons, providing a structured framework for explaining atomic behaviour and nuclear properties.
Structure of the Nuclear Atom
The simple nuclear model provides a conceptual description of atomic structure based on the understanding developed through early twentieth-century experiments. It depicts the atom as being composed of a central nucleus, containing protons and neutrons, with electrons arranged around it in distinct orbitals or shells.
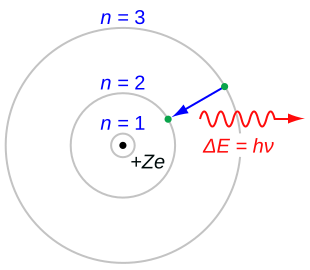
A schematic Bohr model showing a positively charged nucleus with electrons moving in discrete orbits. It highlights the separation between the dense nucleus and orbiting electrons. The ΔE = hν notation is an additional detail beyond this subsubtopic. Source.
This picture replaces earlier uniform-density atomic models and highlights the highly concentrated nature of nuclear mass and charge.
Components of the Nucleus
The nucleus contains two types of nucleons, a collective term used to refer to the particles inside the nucleus. These nucleons determine the atom’s mass and contribute to all nuclear properties.
Nucleon: A constituent particle of the nucleus, specifically a proton or neutron.
The inclusion of both protons and neutrons in the nuclear model provides a framework for explaining nuclear stability, mass behaviour, isotopes, and the balance of forces within the nucleus. While protons carry positive charge, neutrons are electrically neutral, allowing the nucleus to contain multiple like-charged particles without experiencing complete electrostatic disruption, due to additional stabilising nuclear forces. This is explored in more depth elsewhere in the syllabus but underpins the basic model presented here.
Protons
Protons are the positively charged constituents of the nucleus. Their presence defines the element and contributes to both the electric charge and the mass of the atom.
Proton: A positively charged nucleon found in the nucleus, responsible for determining the atomic (proton) number of an element.
Because protons repel one another through electrostatic forces, the presence of neutrons is essential in counteracting these repulsive interactions, enabling larger nuclei to exist.
Neutrons
Neutrons add mass to the nucleus and play a crucial stabilising role. They do not contribute to the overall charge but influence the isotopic form of an element.
Neutron: A neutral nucleon found in the nucleus that contributes to mass and affects nuclear stability through its interaction with protons and other neutrons.
The ratio of neutrons to protons varies across the periodic table. Lighter elements tend to have approximately equal numbers, whereas heavier elements require increasingly more neutrons to remain stable.
Electrons and Atomic Structure
Electrons are fundamental to the atom’s external structure and chemical behaviour. In the simple nuclear model, they are considered to orbit the nucleus in specific regions associated with quantised energy levels.
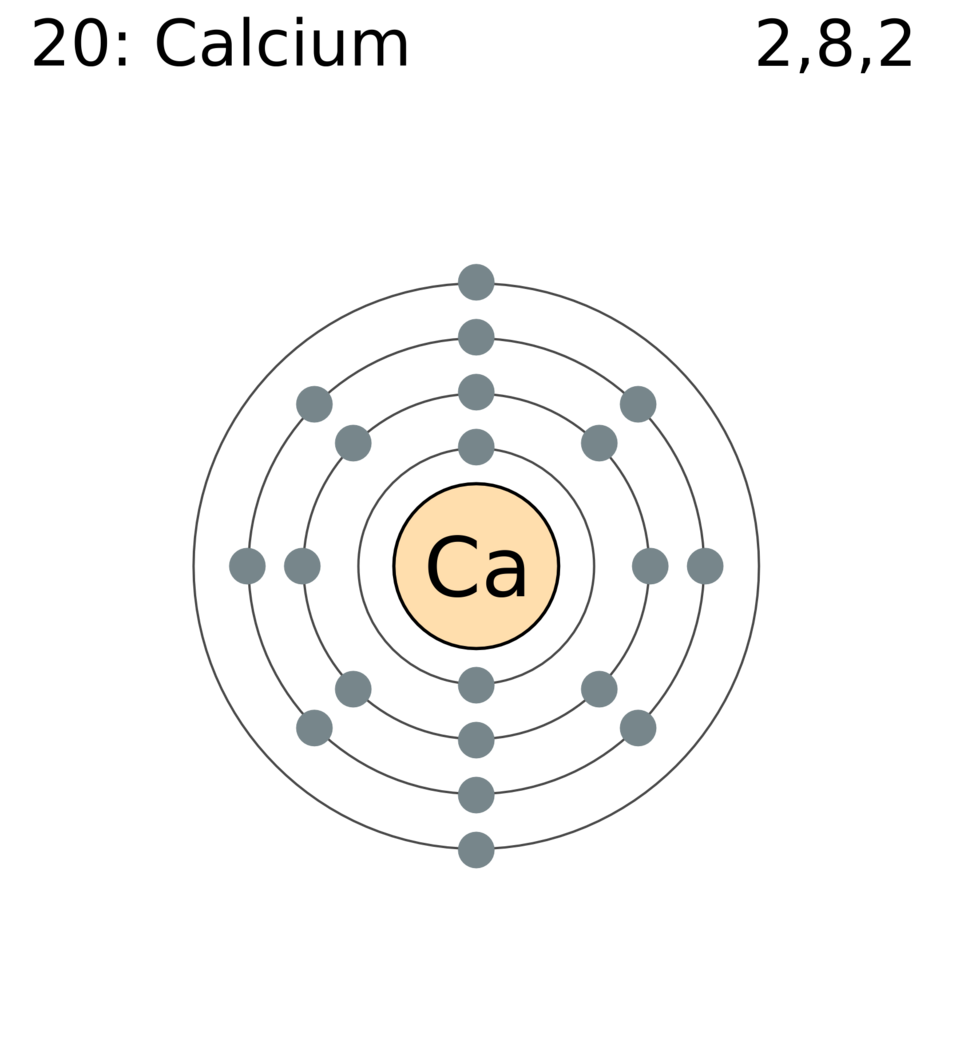
An electron-shell diagram for calcium showing electrons in discrete shells around a central nucleus region. It illustrates the simple nuclear model by depicting electrons orbiting the nucleus. The specific electron configuration shown is additional detail beyond this subsubtopic. Source.
While the model simplifies the distribution of electrons compared with quantum mechanical treatments, it remains adequate for discussing nuclear properties and fundamental atomic principles at this stage.
Properties of Electrons
Electrons carry a negative charge equal in magnitude to the proton’s positive charge. In a neutral atom, the number of electrons matches the number of protons, ensuring electrical neutrality. Their extremely small mass relative to nucleons means they contribute negligibly to total atomic mass, yet they determine the atom’s size, since the electron cloud defines the outer boundary of the atom.
Representation of Atoms Using the Simple Nuclear Model
The model supports a clear way of representing nuclear composition. Each nucleus is defined by its proton number and total number of nucleons. Although the formal nuclear notation is treated in later sections, the simple nuclear model underpins the meaning of these quantities.
A nucleus is therefore described by:
• Number of protons — determines the element
• Number of neutrons — distinguishes isotopes of the same element
• Total nucleon number — gives the mass number
Isotopes and Nuclear Composition
The model naturally leads to the concept of isotopes, since different nuclei of the same element may contain different numbers of neutrons while retaining the same number of protons. This variation accounts for differences in nuclear stability and mass while leaving chemical behaviour unchanged, because the electron configurations are determined primarily by proton number.
Isotope: A nuclide of a given element containing the same number of protons but a different number of neutrons.
These variations in neutron number become especially important in nuclear physics, influencing radioactive behaviour, binding energy, and nuclear reactions discussed elsewhere in the syllabus.
Key Features Emphasised by the Simple Model
The model highlights several crucial characteristics of the nuclear atom:
Mass Concentration
• Nearly all the atom’s mass is concentrated in the nucleus.
• Electrons, while vital to chemical interactions, contribute almost no mass.
• The dense nucleus contrasts sharply with the vast empty space of the surrounding electron cloud.
A brief discussion of the density and scale of the nucleus appears in later subtopics, but it is essential to recognise that this model provides the foundation for those ideas.
Charge Distribution
• Positive charge is confined to the nucleus via protons.
• Negative charge is distributed in the electron cloud.
• The separation of charge explains many atomic behaviours, including ion formation and electrostatic interactions.
Particle Arrangement and Forces
• Protons and neutrons are tightly bound together by nuclear forces.
• Electrons remain in motion around the nucleus due to electrostatic attraction.
• The stability of the configuration depends on a balance between repulsive and attractive interactions, although the simple model does not attempt to quantify these forces.
Applications and Usefulness of the Simple Nuclear Model
Even though more advanced models exist, this simple nuclear model remains an essential conceptual tool in A-Level Physics for understanding:
• Atomic identity and elemental classification
• Nuclear structure and isotopic variation
• The basis of radioactivity and nuclear reactions
• Organisation of the periodic table
• The distinction between atomic and nuclear scales
FAQ
Early models, such as Thomson’s “plum pudding” model, treated charge as spread out uniformly within the atom.
Rutherford’s scattering experiment showed most alpha particles passed through foil undeflected, revealing that nearly all atomic mass and positive charge must be concentrated in a tiny central nucleus.
This experimental evidence provided the basis for the simple nuclear model: a dense nucleus with surrounding electrons rather than a diffuse charge distribution.
The simple model shows electrons orbiting like planets, but this does not reflect their quantum behaviour.
Electrons occupy quantised energy levels rather than fixed circular orbits. Their positions are better described by probability distributions rather than exact paths.
However, the simple model is retained at this level because it helps visualise atomic structure without introducing complex mathematical ideas.
Grouping them as nucleons reflects their shared location in the nucleus and similar masses.
This helps distinguish nuclear structure from atomic structure, where electrons are considered separately.
It also emphasises that many nuclear properties, such as mass number and nuclear stability, depend on the total number of nucleons rather than their individual types.
The simple nuclear model focuses on the nucleus containing protons and neutrons. Because the proton number determines the element, variations in neutron number naturally lead to isotopes.
In this model:
• Same number of protons → same element
• Different numbers of neutrons → different isotopes
Chemical behaviour remains the same because electrons are unchanged, but nuclear stability and mass differ.
The nucleus occupies an extremely small region compared with the overall size of the atom.
Electrons are found at much larger average distances from the nucleus, creating a vast region where no massive particles exist.
This contrast explains:
• Low density of matter at the atomic scale
• Why alpha particles pass through thin metal foil
• Why atoms can be compressed under extreme pressures
Practice Questions
Question 1 (2 marks)
State the key features of the simple nuclear model of the atom.
Mark scheme:
• Atom contains a small, dense nucleus made of protons and neutrons. (1)
• Electrons orbit the nucleus in surrounding shells or energy levels. (1)
Question 2 (5 marks)
Describe the structure of the nucleus and explain why neutrons are necessary within the simple nuclear model. In your answer, refer to charge, stability, and the role of nucleons.
Mark scheme:
• Nucleus contains protons and neutrons (nucleons). (1)
• Protons are positively charged; neutrons are electrically neutral. (1)
• Protons repel each other due to electrostatic repulsion. (1)
• Neutrons contribute to nuclear stability by helping bind nucleons together (via the strong nuclear force). (1)
• Increasing proton numbers require more neutrons to maintain a stable nucleus. (1)

