OCR Specification focus:
‘Compare atomic and nuclear sizes; estimate orders of magnitude of radii.’
Understanding the relative sizes of atoms and their nuclei is essential for appreciating nuclear structure, modelling interactions, and interpreting experimental evidence about matter’s underlying organisation.
Atomic and Nuclear Dimensions
Atoms consist of a central nucleus surrounded by orbiting electrons, yet the nucleus occupies only a minute fraction of the atom’s total volume. Recognising the scale difference between these two regions helps students understand why matter is mostly empty space and why nuclear processes involve such high energies.
The nucleus occupies an extremely small central region, while the electrons are found in a much larger surrounding atomic region.
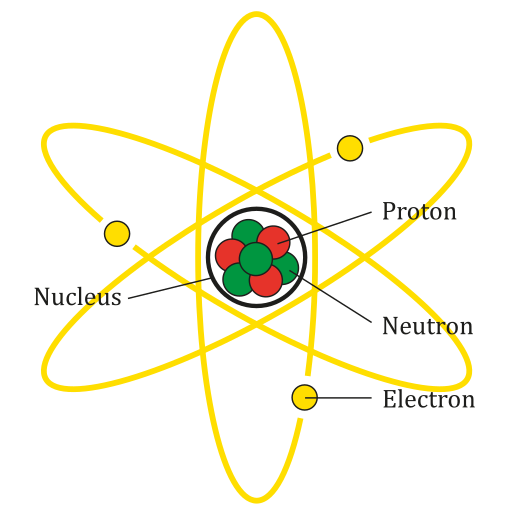
Idealised atomic diagram showing a compact central nucleus surrounded by electrons in discrete shells. Although schematic, it highlights the vast difference between nuclear and atomic radii. Orbital paths are not to scale but reinforce that the atom is mostly empty space. Source.
Typical atoms have radii on the order of 10⁻¹⁰ m, whereas their nuclei have radii on the order of 10⁻¹⁵ m. This contrast represents a difference of about five orders of magnitude. In other words, a nucleus is roughly 100,000 times smaller in radius than the atom it belongs to, meaning the volume ratio is smaller still.
Orders of Magnitude and Scientific Notation
Physics relies on orders of magnitude to compare quantities differing hugely in scale. Here, the nuclear radius is about 10⁵ times smaller than an atomic radius. Because volume scales with the cube of radius, the difference in volume reaches 15 orders of magnitude, reinforcing how concentrated mass and positive charge are at the atomic centre.
Order of magnitude: A comparison expressing how many powers of ten one physical quantity differs from another.
Understanding orders of magnitude enables students to judge the plausibility of physical models and to relate unfamiliar measurements to known reference scales.
A crucial implication of these scale differences is the remarkably low average density of an atom despite the extremely high density of the nucleus. This separation of scale supports the classical picture in which electrons occupy a large region of space while hardly contributing to atomic mass.
Experimental Basis for Size Estimates
The recognition that atoms possess tiny, dense nuclei emerged from the interpretation of scattering experiments. Although this subsubtopic does not focus on Rutherford scattering itself, the comparison of sizes relies on the same experimental reasoning: high-energy alpha particles deflect only when approaching very close to the centre of positive charge, indicating that this region must be extremely small.
The nucleus is a tiny, extremely dense region containing protons and neutrons (nucleons), and it defines the nuclear radius we compare with the atomic radius.
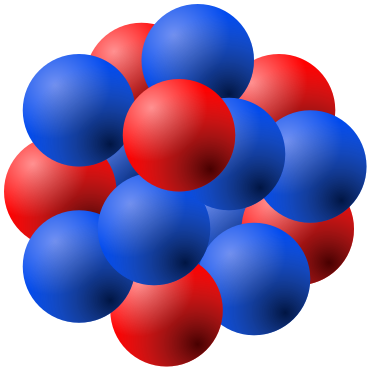
Schematic illustration of a nucleus composed of tightly packed protons and neutrons. It highlights that the nuclear radius refers to this compact region, far smaller than the entire atom. The image includes simplified structural detail beyond the syllabus but reinforces the idea of a dense central nucleus. Source.
Observations supporting relative size estimates include:
Most incident particles pass straight through thin metal foils, implying vast empty space in the atom.
Only a very small fraction experience large deflections, suggesting a compact, central nucleus.
The magnitude of deflection angles correlates with the proximity of the alpha particles to the nucleus, enabling physicists to infer nuclear dimensions.
These findings justify quoting nuclear radii around 1–10 femtometres (fm), where 1 fm = 10⁻¹⁵ m, contrasting sharply with the much larger atomic scale expressed in ångströms (1 Å = 10⁻¹⁰ m).
Conceptual Interpretation of Relative Scale
The extraordinary difference in size between atom and nucleus has several conceptual consequences that students should appreciate:
Mass Concentration
Nearly all atomic mass resides in the nucleus, so a highly localised core contains almost the entire mass of the atom.Charge Distribution
The positive charge is confined to the nucleus, whereas electrons form a cloud constituting most of the atomic radius.Interaction Ranges
Electrostatic forces act over atomic distances and influence bonding, ionisation, and excitation.
Nuclear forces, in contrast, act only within the very small nuclear region and play no direct role in chemical behaviour.
Energy Scales
Energies associated with nuclear processes are far greater than those connected with electronic transitions because the former involve rearrangements of particles within a much smaller spatial region.
These ideas reinforce why comparing atomic and nuclear sizes is a central step toward understanding the boundaries of different physical theories.
Estimating Radii: Useful Relations
Although this subsubtopic does not require detailed derivations, OCR students must recognise how typical nuclear radii can be estimated using empirical relationships.
EQUATION
—-----------------------------------------------------------------
Nuclear Radius (R) = r₀ A^(1/3)
R = Nuclear radius (metres)
r₀ = Constant of proportionality, approximately 1.2–1.5 × 10⁻¹⁵ m
A = Nucleon number (dimensionless)
—-----------------------------------------------------------------
This empirical equation arises from modelling the nucleus as having nearly uniform density. Because volume is proportional to A, the radius must grow with the cube root of the nucleon number.
Even though the atomic radius does not follow a simple relationship comparable to the nuclear case, students should appreciate that it remains broadly within the 10⁻¹⁰ m scale across most elements.
A sentence separating blocks as required ensures the structure remains clear and readable for learners.
Practical Comparisons in Learning
To consolidate understanding of relative sizes, students should be comfortable expressing radii using standard prefix notation and comparing them qualitatively. Key practical points include:
Recognising that
Atomic radius ≈ 0.1 nm (10⁻¹⁰ m)
Nuclear radius ≈ 1–10 fm (10⁻¹⁵ m)
Relating these estimates to the specification requirement: comparing atomic and nuclear sizes and estimating orders of magnitude.
Appreciating that these comparisons are foundational for later topics such as nuclear density, scattering, and the strong nuclear force.
These conceptual skills allow learners to navigate the scale hierarchy governing atomic and nuclear physics and to interpret physical models with confidence.
FAQ
Atomic radii depend mainly on the behaviour of electrons, not the nucleus. Electron shells, shielding, and effective nuclear charge all influence how far electrons are distributed from the nucleus.
In contrast, nuclear radii depend only on the number of nucleons, because nuclear matter has nearly constant density. This gives rise to the simple A^(1/3) scaling, unlike atoms where electron structure introduces irregular trends.
Nuclear sizes are inferred indirectly from experiments such as high-energy scattering and electron diffraction.
In these experiments:
The pattern of deflections or interference provides information about how charge or matter is distributed.
Mathematical models are fitted to the data to extract a characteristic radius that best describes the nuclear structure.
Such techniques allow reliable estimates even though nuclei cannot be imaged optically.
Nuclear matter is bound by the strong nuclear force, which saturates and acts only between close neighbours. This means each nucleon interacts with only a few nearby nucleons, creating a near-constant packing density.
As elements gain more nucleons, they simply add to this compact arrangement without changing the density significantly. This uniformity is what supports the empirical relationship between radius and nucleon number.
Very small nuclei may lack enough nucleons for the attractive nuclear force to overcome electrostatic repulsion between protons.
Very large nuclei contain many protons, increasing repulsive forces and stretching the strong force to its limit. When the balance fails, the nucleus becomes unstable and may undergo radioactive decay.
Thus, nuclear size affects stability not by radius alone but through the balance of forces operating within that volume.
Different interactions operate over different distance scales. Electromagnetic interactions, involving electrons, act across the entire atomic radius, influencing chemistry and bonding.
Nuclear interactions occur only within a few femtometres, so they affect only nucleons inside the nucleus. This scale separation allows distinct models—quantum mechanical for electrons and nuclear/particle models for the nucleus—without interference between the two regimes.
The contrast in size therefore underpins the structure of physics at multiple levels.
Practice Questions
Question 1 (2 marks)
State the approximate radius of an atom and the approximate radius of its nucleus. Use orders of magnitude in your answer.
Question 1 (2 marks)
• Atomic radius stated as around 10⁻¹⁰ m (1 mark)
• Nuclear radius stated as around 10⁻¹⁵ m (1 mark)
Question 2 (5 marks)
Explain why the nucleus must be much smaller than the atom. In your answer:
• refer to experimental observations that reveal the relative sizes
• outline what these observations imply about atomic structure
• discuss how differences in size relate to the distribution of mass and charge within the atom.
Question 2 (5 marks)
Award marks for the following points, up to a maximum of 5:
• Most alpha particles pass straight through thin foils, showing atoms are mostly empty space (1 mark)
• A very small fraction of alpha particles are deflected by large angles, implying a small dense centre (1 mark)
• This centre is identified as the nucleus, whose radius must be orders of magnitude smaller than the atomic radius (1 mark)
• Nearly all the mass is concentrated in the nucleus, reinforcing that it occupies a very small region compared with the atom (1 mark)
• Positive charge is confined to the nucleus, while electrons occupy a much larger region, explaining the difference in scales (1 mark)

