OCR Specification focus:
‘Describe the short-range strong force: attractive to ~3 fm, repulsive below ~0.5 fm.’
Strong Nuclear Force and Its Range
An atomic nucleus remains stable despite intense electrostatic repulsion between positively charged protons, and this stability arises from the strong nuclear force, a short-range interaction. These notes explain how the strong force behaves, why it counteracts repulsion, and how its characteristic range determines nuclear structure and stability.
The strong nuclear force binds protons and neutrons within the nucleus, overcoming electrostatic repulsion through a short-range interaction that becomes attractive at femtometre scales and intensely repulsive at very short distances.
Nature of the Strong Nuclear Force
The strong nuclear force is the fundamental interaction responsible for holding nucleons (protons and neutrons) together inside the nucleus.
Schematic representation of an atomic nucleus showing protons and neutrons packed closely together. Their close proximity reflects the femtometre-scale range of the strong nuclear force. Although forces are not shown explicitly, the diagram reinforces that nucleons must be very close for the strong interaction to act effectively. Source.
It is distinct from both the electrostatic force and the gravitational force, and acts between all nucleons regardless of charge. While the electrostatic force increases dramatically as protons are brought closer, the strong force exhibits a different and highly specific distance dependence.
Strong nuclear force: A short-range fundamental force acting between nucleons, attractive at distances up to roughly 3 femtometres (fm) and repulsive below about 0.5 fm.
The force is extremely powerful at its peak, far stronger than electrostatic attraction or repulsion at equivalent distances. However, its limited range means it becomes negligible just outside the nucleus.
Key Characteristics
The strong nuclear force has several essential properties relevant to A-Level study:
Very short range, significant only within approximately 3 fm.
Charge independence, acting equally between proton–proton, proton–neutron, and neutron–neutron pairs.
Attractive at medium ranges, providing net binding when nucleons are around 1–3 fm apart.
Repulsive at extremely short ranges, preventing nucleons from collapsing into an even denser state.
Much stronger than electrostatic forces over its effective range, ensuring nuclear cohesion.
These characteristics ensure that the nucleus is stable but not crushed into an ultra-dense point.
Distance Dependence and Behaviour
Understanding how the strong nuclear force varies with separation between nucleons is essential.
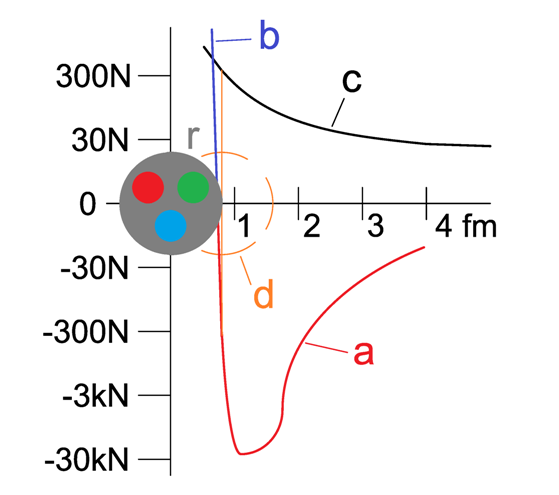
Diagram comparing the nuclear (strong) force and the Coulomb force as a function of distance between nucleons. It highlights the attractive region up to several femtometres and the repulsive core at very short separations. Some additional details such as equilibrium positions are included beyond syllabus requirements but support conceptual understanding. Source.
The behaviour can be described in three key regions.
Region 1: Large Separation (> 3 fm)
At distances greater than approximately 3 fm, the strong nuclear force rapidly falls to zero. This means:
Nucleons separated beyond this range do not attract each other.
The nucleus has a finite size because the force cannot extend sufficiently to bind particles at larger distances.
Stability relies on nucleons being close enough for the attraction to act.
The negligible force beyond this region is why separate nuclei do not spontaneously merge under normal conditions.
Region 2: Intermediate Separation (~0.5–3 fm)
This is the attractive region. Within this distance range:
The force is strongly attractive, overcoming proton–proton electrostatic repulsion.
Nucleons experience the maximum binding effect around 1 fm, a typical spacing within nuclei.
This attraction determines nuclear density and the binding of all stable nuclei.
The near-constant density of nuclei across all elements arises because nucleons pack at roughly this characteristic separation.
Region 3: Very Short Separation (< 0.5 fm)
When nucleons are forced closer than 0.5 fm, the strong nuclear force becomes sharply repulsive. This repulsion:
Prevents nucleons from collapsing into a singular point.
Acts as a “core repulsion” maintaining a minimum separation.
Contributes to the resilience of the nucleus, limiting compression even under extreme conditions.
This repulsive core is essential for nuclear stability and shapes the internal structure of nuclei.
Comparison with Electrostatic Forces
Protons experience electrostatic repulsion, which acts over an infinite range and increases as separation decreases. If electrostatic forces were the only interaction within a nucleus, protons would immediately fly apart.
The strong nuclear force overcomes this problem because:
It becomes overwhelmingly stronger than the electrostatic force within the 1–3 fm attraction region.
Its repulsive core prevents nucleons from collapsing, providing structural balance.
Neutrons, although electrically neutral, contribute significantly to nuclear stability by adding strong-force attraction without increasing electrostatic repulsion.
Neutron-rich isotopes often become more stable because added neutrons strengthen binding without creating additional proton–proton repulsion.
Importance of the Short-Range Nature
The short-range character of the strong nuclear force explains several key nuclear properties:
Finite nuclear size: the force cannot bind particles at large separations.
Constant nuclear density: nucleons arrange themselves at separations where attraction is maximised.
Radioactive instability: unstable nuclei often suffer from imbalances between the strong nuclear force and electrostatic repulsion.
Fusion challenges: overcoming electrostatic repulsion requires extremely high temperatures so nucleons can reach the attractive strong-force region.
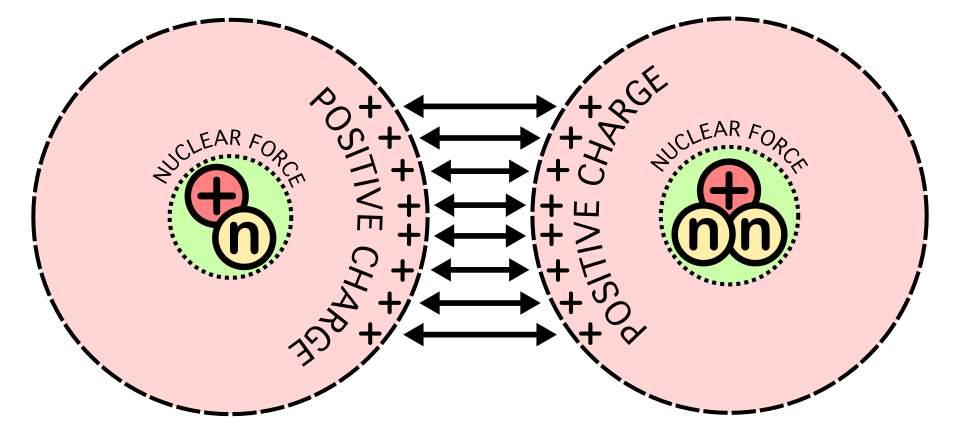
Schematic representation of the forces acting during nuclear fusion, showing Coulomb repulsion at larger separations and the short-range attraction of the strong nuclear force at very small distances. This illustrates the requirement for nuclei to get extremely close before the strong force becomes effective. The diagram includes extra fusion-related details not required by the syllabus. Source.
Range Estimates and Femtometre Scales
Nuclear distances are measured in femtometres (1 fm = 1×10⁻¹⁵ m). Typical values relevant to this subsubtopic include:
Size of a small nucleus: a few femtometres.
Attractive range of strong force: up to ~3 fm.
Repulsive core distance: below ~0.5 fm.
Typical nucleon spacing: around 1 fm.
Working at these extraordinarily small scales emphasises why nuclear forces behave so differently from everyday forces.
Summary of Behaviour Across the Range
To consolidate understanding of how the strong nuclear force governs nuclear structure:
> 3 fm: negligible force, no binding.
0.5–3 fm: strong attraction, maximum stability.
< 0.5 fm: strong repulsion, preventing collapse.
These distinctions provide the foundation for understanding nuclear binding, stability, and structural design in all nuclei.
FAQ
At distances of around 1 femtometre, the strong nuclear force is the dominant interaction, exceeding the electrostatic force by several orders of magnitude.
This dominance disappears rapidly with distance. Beyond roughly 3 femtometres, the strong force becomes so small that even the comparatively weak electrostatic repulsion between protons becomes significant.
Because the gravitational force is negligible at nuclear scales, it plays no role in nucleon interactions.
The strong nuclear force arises from interactions between quarks, which make up both protons and neutrons. These interactions do not depend on electric charge, which is why the force is described as charge-independent.
This symmetry ensures that:
neutron–neutron pairs experience attraction,
proton–proton pairs experience attraction (in addition to electrostatic repulsion),
proton–neutron pairs experience the same strong-force attraction.
The change in behaviour is governed by how the internal quark structures of nucleons interact when they overlap.
At medium separations, the attractive component dominates due to meson exchange between nucleons.
At extremely short distances, the quarks inside nucleons begin to overlap significantly, causing a rapid rise in repulsive interaction that prevents further compression.
This repulsive core stabilises nuclei by ensuring that nucleons cannot collapse into a single point.
The force decreases extremely rapidly with distance because its mediators (such as pions in the simplified model) have mass, limiting the range of the interaction.
Beyond about 3 femtometres:
the probability of exchanging these particles becomes too small,
the force effectively drops to zero,
nuclei behave independently unless driven together by extreme conditions such as high-energy collisions.
Nuclei gain binding energy when nucleons interact attractively, but each nucleon can only interact strongly with its close neighbours.
This leads to:
a roughly constant binding energy per nucleon for medium-sized nuclei,
reduced binding in very small nuclei (fewer close neighbours),
reduced binding in very large nuclei where electrostatic repulsion grows but the strong force cannot reach across the whole nucleus.
The balance between short-range attraction and long-range repulsion shapes overall nuclear stability.
Practice Questions
Question 1 (2 marks)
State two key features of the strong nuclear force that allow the nucleus to remain stable despite electrostatic repulsion between protons.
Question 1 (2 marks)
Award one mark for each correct point, up to two marks.
The strong nuclear force is attractive at distances up to about 3 fm. (1)
The strong nuclear force is stronger than the electrostatic repulsion between protons at this range. (1)
The strong nuclear force acts between all nucleons, not just protons. (1)
The strong nuclear force is repulsive below about 0.5 fm, preventing collapse. (1)
Question 2 (5 marks)
Figure 1 shows the qualitative variation of the strong nuclear force with separation between two nucleons.
Using your knowledge of the strong nuclear force, explain:
(a) why nucleons must be within a few femtometres of each other for the force to bind them,
(b) why the force becomes repulsive at very short distances, and
(c) how the short-range nature of the force influences the size and density of nuclei.
Question 2 (5 marks)
(a) Explanation of binding at a few femtometres:
The strong nuclear force is only significant within approximately 3 fm. (1)
Beyond this distance it becomes negligible, so nucleons must be close to experience strong attraction. (1)
(b) Explanation of repulsion at short distances:
At separations below about 0.5 fm, the force becomes repulsive. (1)
This repulsive core prevents nucleons from collapsing into an extremely dense point. (1)
(c) Influence on nuclear size and density:
The short-range attraction means nucleons pack at roughly 1 fm apart, giving nuclei a nearly constant density. (1)
The finite range of the force limits the overall size of stable nuclei because nucleons beyond a few femtometres cannot be bound. (1)

