OCR Specification focus:
‘Conductors, semiconductors, and insulators differ in number density of charge carriers n.’
Number density links microscopic particle behaviour to macroscopic electrical current. Understanding how it varies across conductors, semiconductors, and insulators is essential for explaining why different materials conduct electricity differently.
Understanding Number Density
Number density, denoted n, refers to the number of charge carriers per unit volume in a material. These charge carriers are typically electrons in metals, or free electrons and holes in semiconductors and insulators. The value of n directly influences how effectively a material can conduct electricity. The higher the number density, the more charge carriers are available to move under an applied electric field, allowing greater current to flow.
Number density (n): The number of free charge carriers per unit volume of a material, measured in m⁻³.
In metals, n is typically extremely large, often around 10²⁸ m⁻³, due to the abundance of free electrons in the metallic lattice. In contrast, semiconductors and insulators possess significantly lower values of n, leading to drastically different electrical behaviours.
Linking Number Density to Electric Current
Number density connects microscopic and macroscopic perspectives of electric current. The mean drift velocity (v) of charge carriers, the cross-sectional area (A) of the conductor, and the elementary charge (e) together determine current according to the continuity equation.
EQUATION
—-----------------------------------------------------------------
Electric current (I) = A n e v
I = current (amperes, A)
A = cross-sectional area of conductor (m²)
n = number density of charge carriers (m⁻³)
e = elementary charge (1.6 × 10⁻¹⁹ C)
v = mean drift velocity of charge carriers (m s⁻¹)
—-----------------------------------------------------------------
From this relationship, it follows that for a given current, materials with smaller number density require their charge carriers to move faster to sustain the same current. Conversely, materials with high number density allow the same current with slower carrier motion.
Classification by Number Density
The OCR specification distinguishes three main material types by number density: conductors, semiconductors, and insulators.
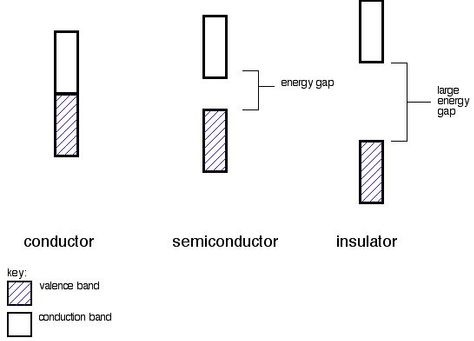
Energy-band structures for a conductor (overlapping bands → abundant carriers), a semiconductor (small band gap → moderate, temperature-dependent carriers), and an insulator (wide band gap → negligible carriers). These differences underpin the much higher n in metals than in semiconductors or insulators. The figure emphasises band occupancy and gap size, which directly ties to carrier availability. Source.
Each category reflects how many free or mobile charge carriers are available to transport electric charge.
Conductors
Conductors, such as copper, aluminium, and silver, have very high number densities. Each atom contributes one or more delocalised electrons, forming a “sea” of mobile charge carriers. These electrons move freely throughout the metallic lattice, enabling efficient electrical conduction.
Typical n value: approximately 10²⁸ m⁻³.
Charge carriers: delocalised (free) electrons.
Electrical behaviour: current flows easily under small potential differences.
Temperature effect: increasing temperature slightly decreases conductivity due to enhanced lattice vibrations causing electron scattering.
Conductor: A material containing a large number of free charge carriers, allowing electric current to flow easily with minimal resistance.
The immense number density in metals explains their use in electrical wiring and components requiring high current flow. The metallic bond structure provides electrons that are not bound to specific atoms, resulting in highly mobile carriers.
Semiconductors
Semiconductors, such as silicon and germanium, have a much smaller number density than conductors. At absolute zero, they behave as insulators because all electrons are bound within covalent bonds. However, at room temperature, some electrons gain sufficient energy to break free, leaving behind positively charged holes. Both the freed electrons and holes act as charge carriers, contributing to electrical conduction.
Typical n value: approximately 10²¹ m⁻³ at room temperature.
Charge carriers: free electrons and holes.
Electrical behaviour: conductivity increases significantly with temperature.
Temperature effect: higher temperature generates more carriers, increasing n and enhancing conductivity.
Semiconductor: A material whose number density and conductivity lie between those of a conductor and an insulator, and which increases conductivity with temperature.
An important feature of semiconductors is their tunable conductivity. Through a process known as doping, minute quantities of impurities are added to control the number density of carriers. This modification is fundamental to modern electronic devices such as diodes and transistors, where precisely controlled carrier densities are critical for operation.
Insulators
Insulators, including glass, rubber, and plastic, possess extremely low number densities. In these materials, electrons are tightly bound to atoms and cannot move freely. Even under high potential differences, very few carriers are available to sustain a current, making them ideal for preventing the flow of electricity.
Typical n value: approximately 10⁶ to 10¹⁰ m⁻³.
Charge carriers: virtually none under normal conditions.
Electrical behaviour: negligible current flow even under large voltages.
Temperature effect: slight increase in n with temperature, though still far too small for significant conduction.
Insulator: A material with extremely low number density of mobile charge carriers, preventing significant electric current from flowing under normal conditions.
Although temperature can release a few charge carriers in insulators, the increase is minimal. Thus, insulators remain essential for providing electrical isolation and protection in circuits.
Comparative Overview of Number Density
The difference in number density across these material classifications can be summarised as a continuum:

Band-filling view of the three material classes. Metal: partially filled/overlapping bands → many mobile electrons (high n). Semiconductor: small gap with Fermi level between bands → few intrinsic carriers at room temperature (moderate n); Insulator: large gap → almost no carriers (very low n). No extra device physics beyond the syllabus is shown. Source.
Conductors: n ≈ 10²⁸ m⁻³ → extremely high carrier concentration.
Semiconductors: n ≈ 10²¹ m⁻³ → moderate, temperature-dependent carrier concentration.
Insulators: n ≈ 10⁶–10¹⁰ m⁻³ → negligible carrier concentration.
The seven orders of magnitude difference between semiconductors and conductors, and the even greater gap between semiconductors and insulators, highlight how strongly material structure determines electrical behaviour. This enormous variation in n underpins all applications of electronic materials, from power transmission to microprocessor design.
Importance in Modern Physics and Technology
Understanding number density allows physicists and engineers to predict and manipulate electrical properties at both atomic and macroscopic scales. In modern technology, control of n defines the behaviour of electronic devices:
In metals, stable high n ensures efficient current conduction.
In semiconductors, adjustable n enables switching, amplification, and digital logic.
In insulators, minimal n maintains safety and separation of conductive elements.
This relationship between microscopic particle density and macroscopic conductivity remains a foundational concept in both physics and electronic engineering, directly linking the motion of individual charge carriers to the performance of electrical systems.
FAQ
The number density in a metal is determined by its atomic structure and the number of delocalised (free) electrons each atom contributes.
Each metallic atom donates one or more electrons to a shared “electron sea.” For example:
Copper and silver each contribute roughly one free electron per atom.
Aluminium contributes three, leading to a slightly higher number density.
This fixed number of delocalised electrons means that, unlike in semiconductors, the number density of metals is essentially constant with temperature.
Doping introduces controlled amounts of impurity atoms to a pure semiconductor crystal to adjust its carrier density.
n-type doping: Adds donor atoms (e.g., phosphorus in silicon), supplying extra electrons and increasing n.
p-type doping: Adds acceptor atoms (e.g., boron), creating “holes” which act as positive charge carriers.
Even small doping levels can increase the number density by many orders of magnitude, transforming a nearly insulating material into one with significant conductivity.
In semiconductors, most electrons are bound in covalent bonds within the crystal lattice, leaving very few free carriers at room temperature.
Only a small fraction of electrons gain enough energy to cross the band gap into the conduction band, creating mobile electrons and holes.
In metals, by contrast, the conduction band is already filled with free electrons even at very low temperatures, resulting in an enormously higher carrier density.
Number density can be calculated indirectly using electrical measurements and known material properties.
Common approaches include:
Measuring current, cross-sectional area, and drift velocity in the equation I = A n e v.
Using Hall effect experiments, which determine charge carrier concentration from the Hall voltage produced in a magnetic field.
These measurements allow physicists to estimate n for different materials without directly counting electrons.
Yes. Number density influences how quickly charge carriers can respond to changing electric fields in AC circuits.
Materials with high n (like metals) can rapidly adjust carrier motion to follow high-frequency signals.
Materials with low n (like semiconductors and insulators) have limited carrier mobility and slower response times.
This affects how materials are used: metals are ideal for AC wiring, while semiconductors are used in controlled-frequency devices such as diodes and transistors.
Practice Questions
Question 1 (2 marks)
Explain why the number density of charge carriers in a semiconductor increases with temperature, whereas in a metal it decreases slightly.
Mark scheme
1 mark: States that in a semiconductor, increasing temperature provides energy to electrons, freeing them from atoms and creating more charge carriers (electrons and holes).
1 mark: States that in a metal, increasing temperature causes more lattice vibrations, leading to greater electron scattering and a slight reduction in effective carrier mobility (or current).
Question 2 (5 marks)
The table below shows approximate number densities of charge carriers (n) for three materials at room temperature:
Copper: 8.5 × 10²⁸ m⁻³
Silicon: 1.0 × 10²¹ m⁻³
Glass: 1.0 × 10⁸ m⁻³
(a) Classify each material as a conductor, semiconductor, or insulator. (3 marks)
(b) Using your answers to part (a), describe and explain how the number density of charge carriers affects the electrical conductivity of these materials. (2 marks)
Mark scheme
(a)
1 mark: Identifies copper as a conductor.
1 mark: Identifies silicon as a semiconductor.
1 mark: Identifies glass as an insulator.
(b)
1 mark: States that materials with higher number density have greater electrical conductivity because more charge carriers are available to move under an electric field.
1 mark: States that lower number density (as in semiconductors and insulators) results in reduced or negligible conductivity due to fewer free carriers available for current flow.

