AP Syllabus focus:
‘Describe how water’s high specific heat capacity helps organisms maintain relatively stable internal body temperatures and homeostasis.’
Water buffers temperature change in cells, tissues, and whole ecosystems. This page explains how high specific heat capacity stabilizes internal conditions, supporting homeostasis and protecting temperature-sensitive biological structures and reactions.
Water’s Thermal Property That Matters Most: High Specific Heat
Water can absorb or release substantial heat with only a small change in its own temperature. This property comes from water molecules forming many hydrogen bonds, which require energy to disrupt.
Specific heat capacity: The amount of heat required to raise the temperature of 1 gram of a substance by 1°C.
In water, added heat energy is often used to weaken or break hydrogen bonds before it increases molecular motion, so temperature rises slowly. Likewise, as water cools, hydrogen bonds reform and release heat, so temperature falls slowly.
Why Hydrogen Bonding Creates Thermal Stability
Because each water molecule can form multiple hydrogen bonds, liquid water behaves like a constantly shifting network.
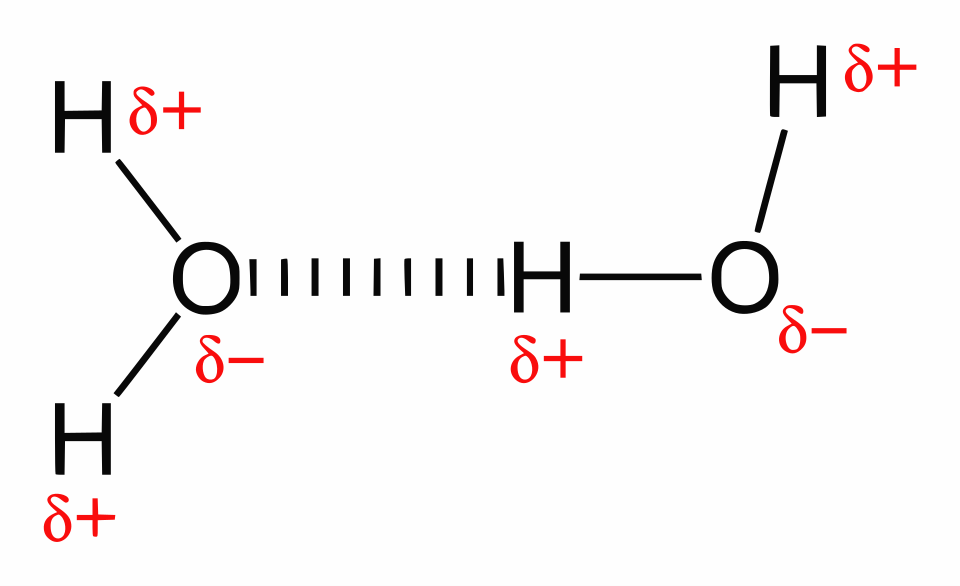
Hydrogen bonding in liquid water is shown as an intermolecular network linking neighboring water molecules. The diagram highlights that hydrogen bonds are continuously breaking and reforming, which helps explain why added heat can be absorbed by bond disruption before producing a large temperature increase. Source
When heat is absorbed:
Energy goes into disrupting hydrogen bonds first.
Less energy immediately converts to faster molecular motion (temperature increase).
When heat is released:
Hydrogen bonds reform, releasing stored energy gradually.
Cooling is moderated, resisting sudden temperature drops.
This does not stop temperature change; it slows the rate of change, which is crucial for living systems.
How High Specific Heat Supports Homeostasis
Homeostasis depends on keeping internal conditions within tolerable ranges. Temperature affects nearly every biological process, so organisms benefit from water’s ability to buffer heat.
Stabilising Body Temperature in Organisms
Many organisms are mostly water (cytosol, blood plasma, interstitial fluid). These aqueous compartments act as heat reservoirs.
Internal fluids absorb heat produced by metabolism, reducing spikes in body temperature.
During cooler periods, stored heat in body water is released, reducing rapid cooling.
More stable temperature helps keep enzyme-catalysed reactions running near their functional ranges, since reaction rates and protein structure are temperature-sensitive.
In multicellular organisms, water-based fluids distribute heat across the body:
Blood (high water content) transports heat from active tissues (e.g., muscle) to other regions or to surfaces where heat can be lost, smoothing local temperature differences.
Protecting Cells and Membranes
Temperature shifts can alter membrane fluidity and disrupt protein shape. Water’s high specific heat reduces the likelihood of rapid temperature swings that would:
impair membrane transport processes,
destabilise protein interactions,
disturb reaction networks that rely on consistent conditions.
Connecting the Concept to Heat Transfer Language
AP Biology may describe heat exchange in terms of absorbed or released thermal energy.
I
= heat energy absorbed or released (J)
= mass of the substance (g)
= specific heat capacity (J/g·°C)
= change in temperature (°C)
This relationship supports the key idea: for a given heat input, a higher c produces a smaller ΔT, so water resists temperature change.
Biological and Environmental Implications of Thermal Buffering
Aquatic and Coastal Temperature Stability
Because large bodies of water absorb heat during warm periods and release it during cool periods:
Aquatic habitats experience less extreme temperature fluctuations than land.
Organisms in water often face a more stable thermal environment, aiding consistent metabolic function.
Coastal regions tend to have narrower temperature ranges than inland areas, influencing organism distribution and seasonal stress.
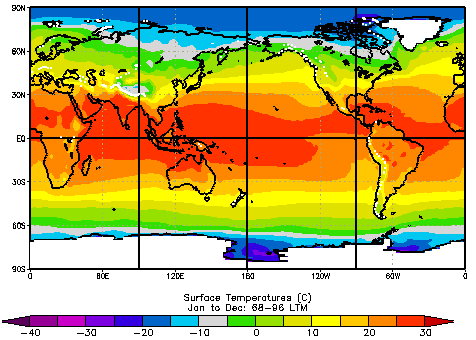
This map shows the average annual temperature range (warmest month minus coldest month) across Earth’s surface. Coastal regions near large water bodies generally have smaller annual temperature ranges than continental interiors, reflecting water’s high heat capacity and its ability to moderate seasonal temperature swings. Source
Organismal Heat Balance Over Time
High specific heat does not generate heat; it moderates heat gain and loss.
In daytime or during intense activity, water-rich tissues absorb excess heat.
At night or in cooler conditions, those tissues release heat more slowly. This buffering supports relatively stable internal body temperatures and contributes to maintaining homeostasis, especially when external temperatures change quickly.
FAQ
Dissolved salts generally lower specific heat capacity slightly by altering how water molecules interact, so seawater warms and cools a bit faster than pure water, though it still buffers temperature strongly.
Large animals generate substantial metabolic heat. High water content in blood and tissues absorbs and redistributes this heat, preventing dangerous local temperature spikes and helping maintain stable core temperatures.
They heat a known mass of a substance by a measured energy input and record the temperature change. Comparing water to another liquid shows how much less water’s temperature rises for the same heating.
Yes. Lower body water reduces thermal buffering capacity, so temperature can change more rapidly. This can increase physiological stress during heat exposure or rapid cooling.
Because water-rich tissues resist temperature change, raising body temperature during fever requires sustained heat production. This makes fever a regulated, energy-demanding process rather than an immediate temperature spike.
Practice Questions
Explain how water’s high specific heat capacity helps an organism maintain a stable internal temperature. (2 marks)
States that water absorbs/releases large amounts of heat with little temperature change (1)
Links this to reduced fluctuations in body temperature/homeostasis (1)
A small mammal’s body temperature remains relatively constant despite a rapid drop in external temperature. Describe how water’s thermal properties contribute to this stability. (5 marks)
Identifies high specific heat capacity of water in body fluids/tissues (1)
Explains that cooling causes water to release heat with only a small decrease in its own temperature (1)
Links to hydrogen bonding (energy stored/released as bonds break/form) (1)
Explains reduced rate of internal temperature change (buffering) (1)
Connects stable temperature to maintaining homeostasis of enzyme-controlled metabolism/physiological function (1)

