AP Syllabus focus:
‘Explain why living systems depend on water’s polarity, hydrogen bonding, and related emergent properties to sustain life.’
Water is not just a background fluid in biology: its molecular structure generates properties that make metabolism possible, stabilize internal conditions, and support organization from cells to ecosystems. These benefits trace back to polarity and hydrogen bonding.
Water’s Structure as the Source of Biologically Useful Properties
Polarity and Hydrogen Bonding Drive “Emergent” Behavior
Because the O–H bonds are polar covalent, water molecules have partial charges that let them attract each other and many other molecules.
Hydrogen bond: A weak attraction between a partially positive hydrogen atom (bound to an electronegative atom) and a partially negative electronegative atom (often oxygen or nitrogen).
A single hydrogen bond is weak, but the collective network of many bonds gives water unusually strong bulk properties that biological systems depend on.
Why “Emergent Properties” Matter to Life
Water’s key life-supporting properties are “emergent” because they arise from interactions among many water molecules, not from one molecule alone. In organisms, these properties help:
Maintain stable internal environments despite external change
Provide efficient chemical transport and mixing
Create physical microenvironments (surfaces, compartments, and gradients) used by cells and tissues
Water as a Solvent: Enabling Chemistry, Transport, and Cellular Organisation
Dissolving and Transporting Essential Substances
Water’s polarity makes it an excellent solvent for ions and polar molecules, surrounding them with hydration shells that keep them dispersed and mobile. This supports life by enabling:
Nutrient and waste transport (e.g., ions, sugars, amino acids) in blood, cytosol, xylem/phloem sap
Diffusion and osmosis, which move materials across short distances and help regulate cell volume
Rapid mixing so reactants collide frequently enough for metabolism to proceed efficiently
Supporting Biochemical Reactions
Many cellular reactions require an aqueous environment because:
Reactants must be in solution to interact with enzymes and substrates
Water stabilizes charged intermediates and helps control local pH through dilution and buffering interactions
Water participates directly in reactions (for example, as a reactant or product in many metabolic pathways), which helps couple chemistry to cellular function without needing extreme temperatures or pressures
Water’s Thermal Properties: Protecting Enzyme Function and Homeostasis
Temperature Stability in Organisms and Habitats
Hydrogen bonding causes water to resist temperature change, which benefits life because proteins and membranes function within narrow temperature ranges. Biologically, this helps:
Keep body temperatures and cellular temperatures more stable, reducing sudden changes in enzyme activity
Moderate environmental temperatures in aquatic and coastal habitats, lowering thermal stress on organisms and stabilising food webs
Preventing Rapid Overheating in Active Tissues
When cells release heat during metabolism, water’s bonding network absorbs substantial energy before temperature rises, limiting thermal spikes that could disrupt:
Protein shape (and therefore enzyme activity)
Membrane fluidity, which affects transport and signalling
Cohesion and Adhesion: Building Biological Transport Systems and Surfaces
Cohesion Supports Continuous Water Columns
Water molecules stick to each other via hydrogen bonds (cohesion), which is essential for moving water through narrow biological spaces. This contributes to:
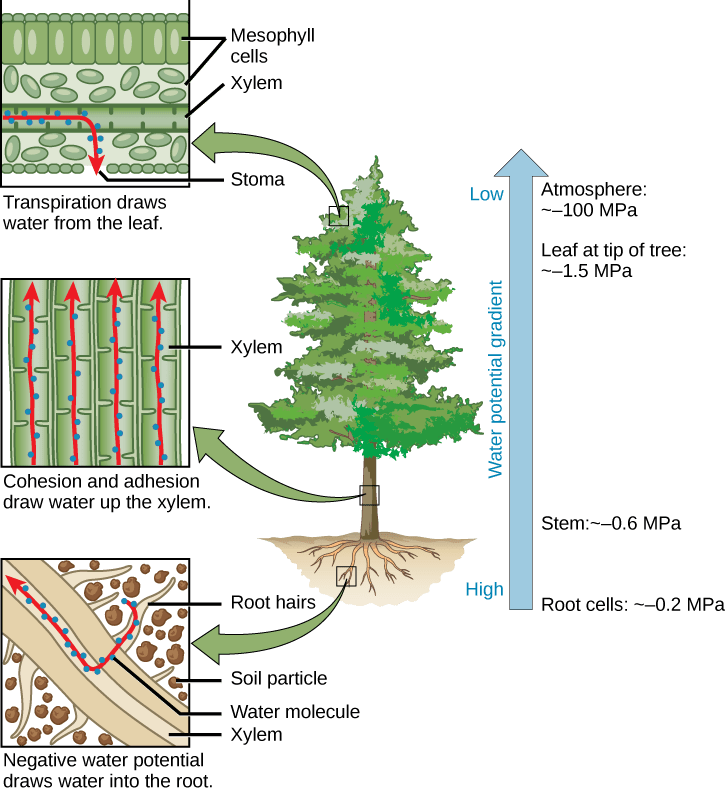
This diagram summarizes the cohesion–tension mechanism of water transport in plants. Transpiration at the leaf generates negative pressure (tension) that pulls a continuous column of water upward through xylem, while cohesion (water–water hydrogen bonding) and adhesion (water–cell wall interactions) help maintain that column against gravity. Source
Maintaining continuous columns of water in plant vascular tissues, supporting long-distance transport
Sustaining surface tension, creating stable interfaces that some organisms exploit for movement, feeding, or gas exchange at water surfaces
Adhesion Supports Wetting and Capillary Effects
Water also sticks to other polar surfaces (adhesion), helping it spread and rise within small channels.
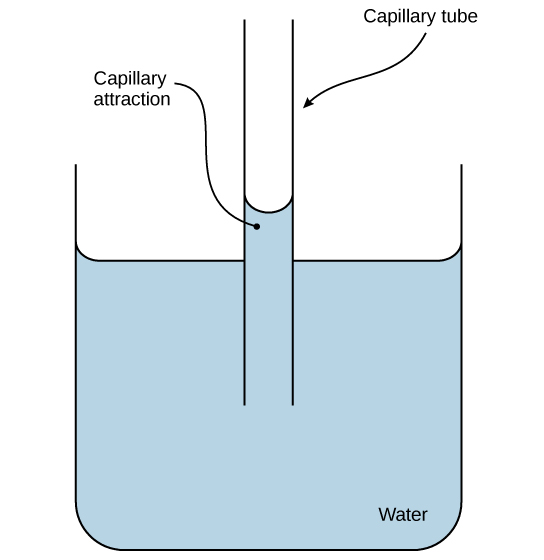
This capillary-action diagram illustrates how adhesion to a polar surface (the tube wall) and cohesion within water (hydrogen bonding) produce a curved meniscus and a net upward rise in a narrow tube. The same physical principle contributes to water movement through microscopic channels in biological tissues, especially alongside transpiration-driven flow in plants. Source
This supports:
Movement along cell walls and extracellular matrices
Distribution of water across moist respiratory and digestive surfaces, aiding diffusion and protecting tissues from drying
Water’s Solid Form and Ecological Stability
Ice as a Protective Layer
Hydrogen bonding makes solid water less dense than liquid water.
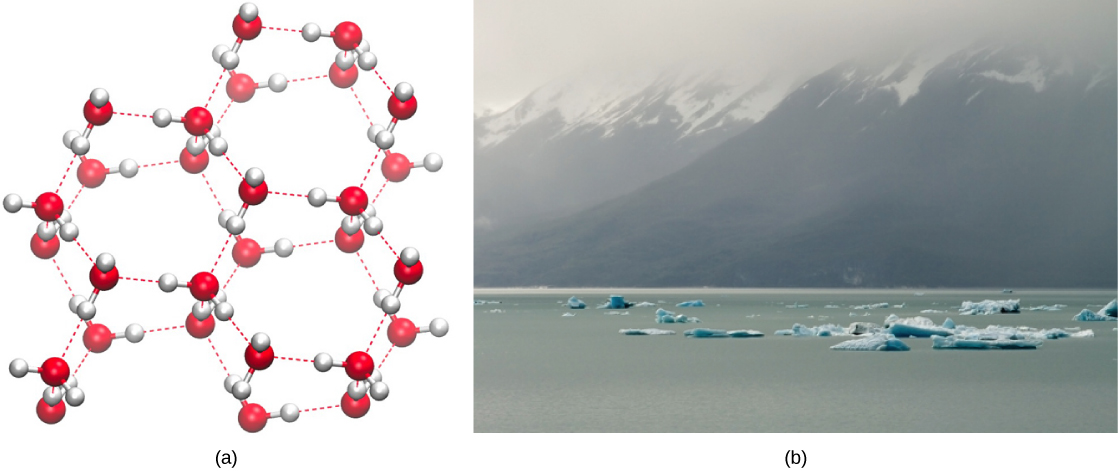
This figure contrasts the open lattice of ice with the more closely packed arrangement of liquid water. When hydrogen bonds lock water molecules into a rigid lattice, the structure contains more empty space, lowering density so ice floats—creating an insulating surface layer that helps stabilize aquatic habitats in cold conditions. Source
As a result, ice floats and forms an insulating surface layer that:
Reduces heat loss from underlying water
Helps aquatic organisms survive cold periods by preserving liquid habitats beneath ice
Linking Properties to Survival: Why Life Depends on Water
Living systems depend on water because polarity and hydrogen bonding collectively provide:
A versatile solvent medium for transport and reaction chemistry
Thermal buffering that protects enzyme-driven metabolism and homeostasis
Mechanical and surface behaviours (cohesion/adhesion/surface tension) that enable biological transport and stable interfaces
Ecological resilience, including stable aquatic habitats across seasonal temperature changes
FAQ
Dissolved ions alter water structure and influence hydration shells. This can shift protein stability and change how readily water moves by osmosis.
Polarity alone is not enough; large size, limited charge separation, or strong internal bonding can reduce interaction with water, lowering solubility.
Transient hydrogen bonds continually break and reform, allowing rapid diffusion while still organising local structure around solutes and surfaces.
Yes. Some insects distribute weight across the surface film, and many small organisms rely on stable air–water interfaces for feeding or gas exchange.
It stabilises whole freshwater ecosystems by limiting winter mixing and preserving under-ice liquid zones, which helps maintain oxygen and habitat continuity.
Practice Questions
Describe how hydrogen bonding between water molecules contributes to one emergent property that supports life. (2 marks)
Identifies a correct emergent property linked to hydrogen bonding (e.g., cohesion, surface tension, thermal buffering, solvent behaviour) (1)
Explains how hydrogen bonding produces that property and why it supports life (1)
Explain why water’s polarity and hydrogen bonding are essential for maintaining living systems, referring to transport, temperature stability, and at least one additional emergent property. (6 marks)
States that polarity allows water to dissolve ions/polar molecules (1)
Links dissolving to transport or availability of reactants for metabolism (1)
Explains that hydrogen bonding gives water thermal buffering (high resistance to temperature change) (1)
Links thermal buffering to homeostasis/enzyme function (1)
Describes one additional emergent property caused by hydrogen bonding (cohesion/adhesion/surface tension or ice floating) (1)
Links that additional property to a biological advantage (e.g., plant water transport, stable surfaces, aquatic survival under ice) (1)

