AP Syllabus focus:
‘Explain differences between saturated and unsaturated fatty acids, including single versus double carbon–carbon bonds and resulting kinks in the hydrocarbon chain.’
Fatty acids differ in whether their hydrocarbon chains contain only single C–C bonds or include C=C double bonds. These bonding differences change chain shape, packing, and many observable properties of fats and oils in biological systems.
Core Idea: Bond Type Changes Shape
Fatty acids are long hydrocarbon chains with a terminal carboxyl group; most structural variation occurs along the carbon chain. The key comparison is single versus double carbon–carbon bonds.
Saturated Fatty Acids (No Double Bonds)
Saturated fatty acid: A fatty acid whose hydrocarbon chain contains only single carbon–carbon (C–C) bonds, so the chain is fully “saturated” with hydrogen atoms.
With only single bonds, carbon atoms can rotate freely around C–C bonds. This usually produces an overall straight chain that can align closely with neighboring fatty acid tails.
A straight chain shape matters because:
Tight packing is easier when many tails are similarly straight.
Close alignment increases van der Waals (London dispersion) interactions between tails.
Stronger collective attractions generally raise the melting point, so these fats are more often solid at room temperature.
Unsaturated Fatty Acids (One or More Double Bonds)
Unsaturated fatty acid: A fatty acid whose hydrocarbon chain contains at least one carbon–carbon double bond (C=C), meaning it is not fully saturated with hydrogen.
A C=C double bond restricts rotation and fixes the geometry around the double bond. In most biological fatty acids, the double bond is cis, which introduces a bend (“kink”) in the hydrocarbon chain.
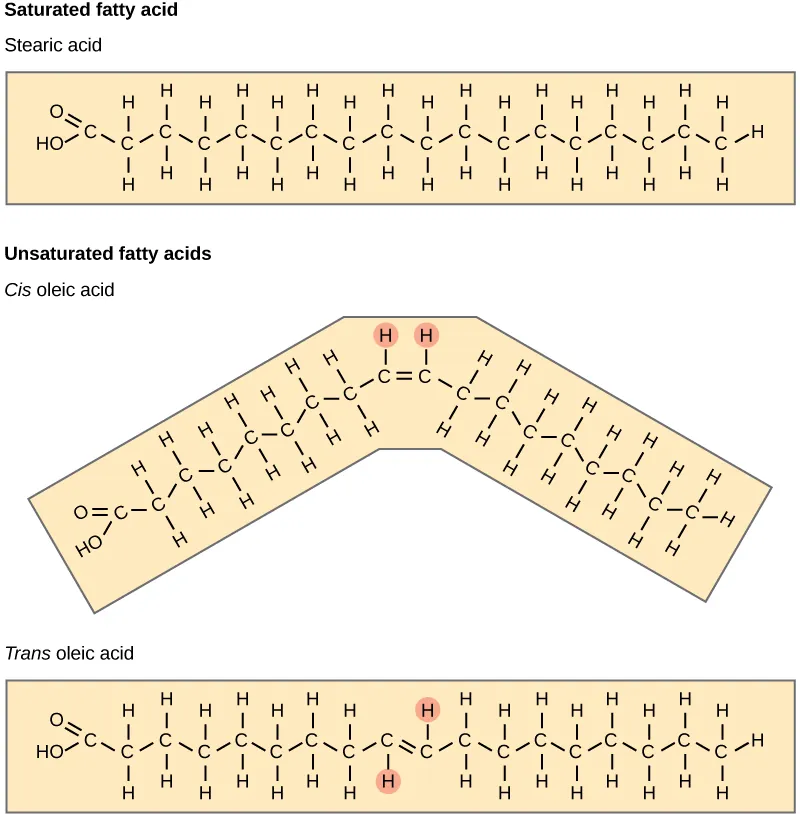
This figure compares a saturated fatty acid (stearic acid) with unsaturated fatty acids that have a C=C double bond in either the cis or trans configuration. The cis double bond produces a pronounced bend (“kink”) in the hydrocarbon chain, while the trans configuration keeps the chain more linear and saturated-like. This directly links bond type and geometry to chain shape, which is the key structural reason unsaturated tails pack less tightly. Source
That kink is the central structural consequence emphasized in AP Biology: double bonds → kinks.
Because kinked tails cannot align as closely:
Packing is less tight between neighboring tails.
Van der Waals interactions are reduced overall.
Melting points tend to be lower, so these fats are more often liquid at room temperature (common in plant oils).
Types of Unsaturation and “Kinks”
Unsaturated fatty acids may be:
Monounsaturated: one C=C double bond (one kink).
Polyunsaturated: two or more C=C double bonds (multiple kinks, typically increasing disorder).
Each additional double bond typically adds another bend, compounding the packing disruption. In drawings, saturated tails appear like straight lines, while unsaturated tails show one or more noticeable angles at double-bond positions.
Cis vs Trans (Why “Kink” Usually Means Cis)
Most naturally occurring unsaturated fatty acids in organisms have cis double bonds, which place hydrogens on the same side of the double bond, creating a bend in the chain. By contrast, trans double bonds position hydrogens on opposite sides, making the chain more linear and saturated-like in shape.
Cis double bond: A C=C double bond arrangement that places substituents on the same side, producing a bend (kink) in a fatty acid chain.
This stereochemistry detail matters because the AP focus links double bonds to the resulting kinks; that relationship is most consistent for cis unsaturation.
Recognising Saturated vs Unsaturated in Shorthand
Biologists often describe fatty acids using carbon count and number of double bonds, e.g.:
############################
page_url: https://www.lipidmaps.org/lipid_nomenclature/fatty_acyls/fatty_acids
image_identifier: ‘Hierarchical shorthand notation for examples of fatty acids’ table (on-page table)
This table from LIPID MAPS illustrates hierarchical fatty-acid shorthand notation (e.g., FA 18:0, FA 18:1, FA 18:2) and extends it to show how double-bond position and geometry can be specified (e.g., 9Z). It reinforces that the second number in the shorthand tracks the number of C=C double bonds, which predicts the likelihood and extent of kinking. Using a standardized nomenclature reference also helps prevent confusion when comparing monounsaturated vs polyunsaturated examples.
############################
18:0 = 18 carbons, 0 double bonds (saturated)
18:1 = 18 carbons, 1 double bond (unsaturated)
18:2 = 18 carbons, 2 double bonds (polyunsaturated)
The presence/absence of double bonds is the defining feature, and the number of double bonds predicts the likelihood and extent of chain kinking.
What AP Biology Expects You to Explain
To meet the syllabus requirement, you should be able to state clearly:
Saturated fatty acids have single C–C bonds only and therefore have straight hydrocarbon chains.
Unsaturated fatty acids have one or more C=C double bonds, which usually create kinks in the hydrocarbon chain.
The kink arises because the double bond restricts rotation and fixes the chain geometry.
Structural shape differences lead to different packing behavior (tight vs loose), which helps explain common physical observations (more solid vs more liquid).
FAQ
Most biological double bonds are cis and do create a bend.
Trans double bonds are more linear and may not noticeably kink the chain.
They can occur at various positions depending on the fatty acid.
The position affects where the bend appears, changing the overall shape.
Look for C=C double bonds along the hydrocarbon chain.
No C=C means saturated; one or more C=C means unsaturated.
A C=C double bond has a rigid planar region due to the pi bond.
This prevents free twisting that would otherwise straighten or rearrange the chain.
No. “Unsaturated” means at least one double bond.
“Polyunsaturated” means two or more double bonds in the same fatty acid chain.
Practice Questions
State one structural difference between saturated and unsaturated fatty acids. (2 marks)
Saturated fatty acids have only single C–C bonds / no C=C bonds. (1)
Unsaturated fatty acids have one or more C=C double bonds. (1)
Explain how carbon–carbon double bonds in unsaturated fatty acids alter the shape of the hydrocarbon chain compared with saturated fatty acids, and how this affects how the chains pack together. (5 marks)
Saturated fatty acids contain only single C–C bonds, allowing a straighter chain. (1)
Unsaturated fatty acids contain at least one C=C double bond. (1)
Double bonds restrict rotation around the bond. (1)
This typically produces a bend/kink in the chain (cis). (1)
Kinked chains pack less closely/less tightly than straight chains. (1)

