AP Syllabus focus:
‘Describe how increasing numbers of double bonds make fatty acid tails more unsaturated and cause lipids to be more liquid at room temperature.’
Lipids vary in physical state because the structure of their fatty acid tails changes how tightly molecules pack. For AP Biology, connect double bonds to unsaturation and then to lipid fluidity.
Key Idea: Double Bonds Change Packing
Fatty acids are hydrocarbon chains; their physical behavior depends on how straight those chains are and how strongly they attract each other through weak intermolecular forces.
Degree of unsaturation: The number of carbon–carbon double bonds in a fatty acid tail; more double bonds means a higher degree of unsaturation.
As the degree of unsaturation increases:
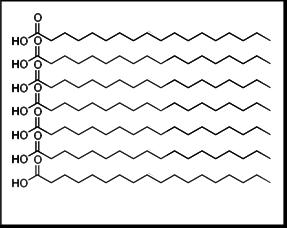
A set of saturated fatty acid chains is depicted as relatively straight hydrocarbon tails that can sit closely side-by-side. This tight packing maximizes surface contact and strengthens the combined weak attractions between tails. As a result, lipids enriched in saturated tails tend to have higher melting points and are more solid-like at room temperature. Source
the tail contains more C=C double bonds
the tail becomes less able to align closely with neighboring tails
lipid molecules pack less tightly, weakening overall tail-to-tail attractions
How Unsaturation Increases Fluidity at Room Temperature
Structural basis
Most biologically common double bonds in fatty acids are cis (same-side) configurations, which introduce a noticeable bend.
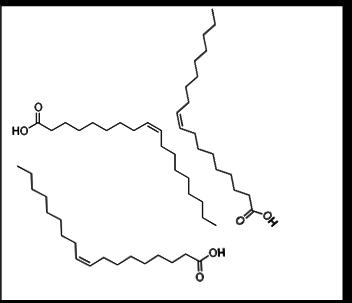
Oleic acid molecules are shown with a cis carbon–carbon double bond that introduces a bend in each hydrocarbon chain. This geometric kink reduces how closely neighboring tails can align, which weakens the cumulative intermolecular attractions in a lipid sample. In biological lipids, this cis-induced bending is a key structural basis for increased fluidity. Source
These bends matter because they:
disrupt the formation of a neat, tightly packed array of hydrocarbon chains
reduce the total surface contact between neighboring tails
lower the cumulative strength of weak attractions between tails
Physical Consequence: “More Liquid at Room Temperature”
When packing is disrupted, less thermal energy is needed to keep lipid molecules moving past one another. Therefore, lipids with more unsaturated tails tend to be more fluid and more liquid at room temperature.
In practical terms:
more double bonds → more kinks/bends → less tight packing → greater fluidity
polyunsaturated (many double bonds) lipids typically have lower melting points than those with few or no double bonds
Comparing Low vs High Unsaturation (What to Say on an Exam)
Low Degree of Unsaturation
fewer (or zero) double bonds
straighter tails can align closely
stronger combined tail-to-tail attractions
higher tendency to be solid or semi-solid at room temperature
High Degree of Unsaturation
more double bonds per tail
more bends that prevent close alignment
weaker combined attractions
higher tendency to be liquid at room temperature
Why this Property Matters Biologically (Fluidity as a Functional Trait)
Although AP Biology often discusses membranes elsewhere, the core idea here is that lipid physical state depends on unsaturation. Cells benefit from lipids that can remain appropriately fluid under typical environmental temperatures.
Higher unsaturation supports fluid-like behavior by:
preventing lipids from locking into rigid, highly ordered arrangements
maintaining molecular movement needed for normal lipid behavior in biological systems
FAQ
No. Cis double bonds create a stronger bend, disrupting packing more and increasing fluidity.
Trans double bonds keep the chain relatively straight, so packing can be tighter, making the lipid behave more like a less unsaturated fat.
Double bonds create reactive sites where oxygen can attack.
With more double bonds, there are more vulnerable positions, increasing the chance of lipid peroxidation and off-flavours/odours.
Many organisms increase the proportion of unsaturated fatty acids in colder conditions.
This helps prevent lipids from becoming too rigid as temperature drops, supporting normal molecular movement.
It is a measure of how much iodine reacts with a lipid sample.
Because iodine adds across double bonds, higher iodine values indicate more double bonds and therefore a higher degree of unsaturation.
Not always. Increased unsaturation generally increases lipid movement, but permeability also depends on factors like tail length, temperature, and the types and amounts of other membrane components.
Practice Questions
Explain how increasing the number of double bonds in a fatty acid tail affects whether a lipid is liquid at room temperature. (2 marks)
States that more double bonds increases the degree of unsaturation and introduces bends/kinks. (1)
Explains that kinks reduce tight packing/weakens attractions between tails, increasing fluidity so the lipid is more liquid at room temperature. (1)
A student compares two lipids: Lipid A contains fatty acid tails with one double bond each, and Lipid B contains tails with three double bonds each. Describe and explain which lipid is expected to be more liquid at room temperature. (5 marks)
Identifies Lipid B as having a higher degree of unsaturation (more double bonds). (1)
Links more double bonds to more bending/kinks in the tails. (1)
Explains that bending reduces close alignment/packing of fatty acid tails. (1)
Explains that reduced packing lowers the overall strength of weak attractions between tails. (1)
Concludes that Lipid B has greater fluidity and is therefore more liquid at room temperature than Lipid A. (1)

