AP Syllabus focus:
‘Describe the 5’ and 3’ ends of nucleic acids and how nucleotides are added to the 3’ end during synthesis.’
Nucleic acids have built-in directionality because their nucleotides link in a consistent chemical orientation. Understanding the 5’ and 3’ ends explains how enzymes extend DNA or RNA strands and why sequences are always written one way.
Nucleic Acid Strand Directionality
What “5’” and “3’” Mean Chemically
The labels 5’ (five-prime) and 3’ (three-prime) refer to numbered carbons in the pentose sugar of each nucleotide (deoxyribose in DNA, ribose in RNA). These numbers help track which end of a strand has which functional group available for bonding.
5’ end: The end of a nucleic acid strand where the terminal nucleotide has a free phosphate group attached to the sugar’s 5’ carbon.
The 5’ end often bears a phosphate, while the opposite end exposes a hydroxyl group that is essential for chain growth.
3’ end: The end of a nucleic acid strand where the terminal nucleotide has a free hydroxyl group (-OH) on the sugar’s 3’ carbon.
Because these ends are chemically distinct, a strand has polarity (a “direction”), and sequences are conventionally written from 5’ to 3’.
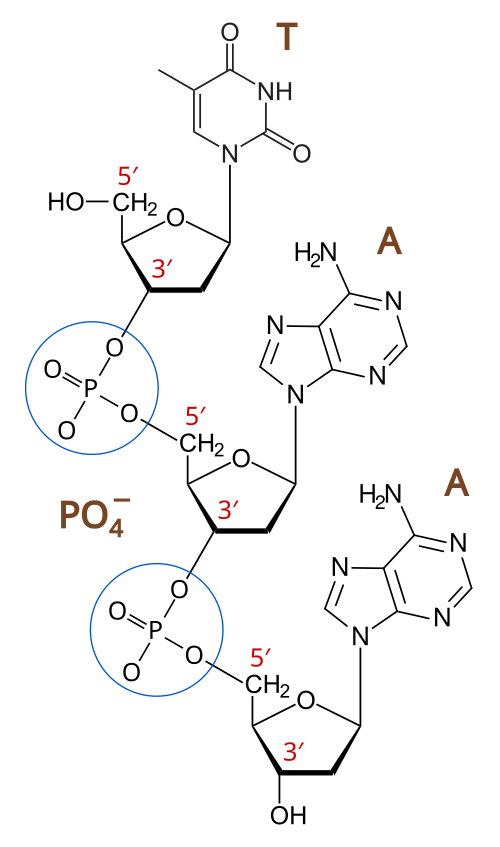
Phosphodiester bonds link nucleotides into a sugar–phosphate backbone, giving the strand chemical polarity. The diagram highlights how the terminal phosphate defines the 5′ end, while the terminal 3′-hydroxyl group defines the 3′ end—features that determine how we label and read nucleic acid strands. Source
The Repeating Backbone and Its Linkage
Nucleic acids are polymers with a sugar–phosphate backbone. Adjacent nucleotides are connected through a covalent bond that links the 3’ carbon of one sugar to the phosphate on the 5’ carbon of the next nucleotide.
Phosphodiester bond: The covalent bond in the sugar–phosphate backbone that forms between the 3’ hydroxyl of one nucleotide and the 5’ phosphate of another nucleotide.
This consistent linkage produces an alternating sugar–phosphate chain with a defined 5’ end and 3’ end.
How Nucleotides are Added during Synthesis
Core Rule: Extension Occurs at the 3’ end
During nucleic acid synthesis, new nucleotides are added to the 3’ end of the growing strand. This means the strand elongates in the 5’ → 3’ direction.
Key idea to memorise: polymerases extend from the 3’ end by using the free 3’ -OH.
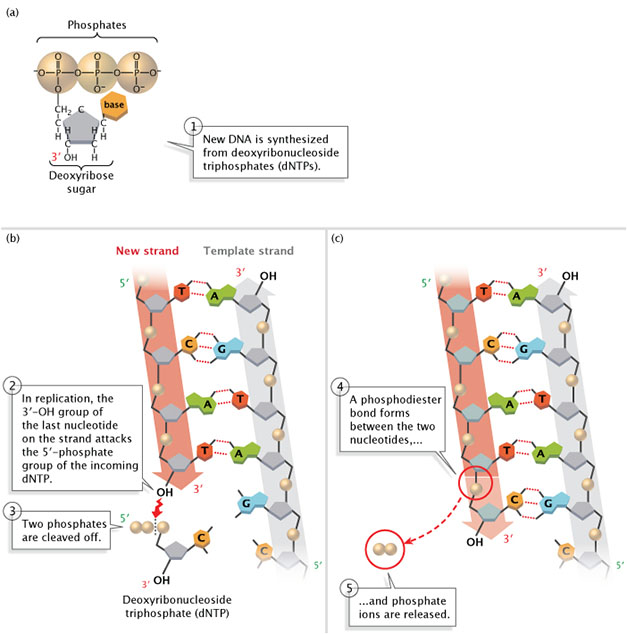
This schematic illustrates why polymerases can only extend a nucleic acid at the 3′ end. The free 3′-OH of the growing strand forms a new phosphodiester bond with the 5′ phosphate of an incoming nucleotide triphosphate, with release of pyrophosphate driving the reaction forward. Source
What the Enzyme Needs to Add a Nucleotide
To add a nucleotide, the synthesis machinery relies on two chemical features:
A free 3’ hydroxyl (-OH) on the last nucleotide of the growing strand (the attachment point)
An incoming nucleotide that carries usable phosphate energy (biologically, nucleotides are supplied in activated forms)
This chemistry ensures that extension can only happen where a 3’ -OH is available, so nucleic acids cannot be “built backwards” from the 5’ end.
What Changes when a Nucleotide is Incorporated
When a nucleotide joins the chain:
The incoming nucleotide becomes the new terminal nucleotide
The chain’s new free 3’ -OH is now on the recently added nucleotide
The strand length increases by one nucleotide while maintaining the same 5’ → 3’ orientation
Because the 3’ end is always the site of addition, diagrams of synthesis typically show growth occurring at the strand’s 3’ tip.
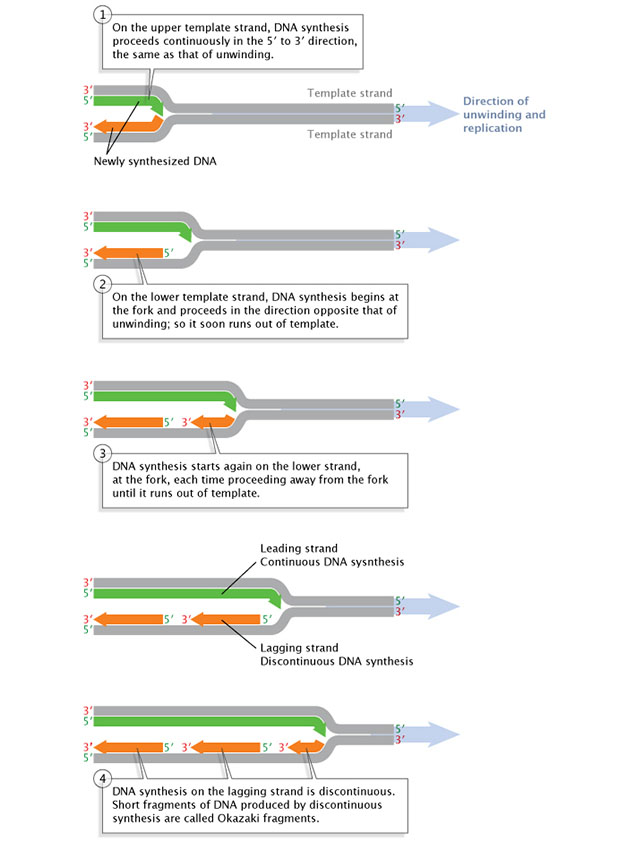
A replication-fork schematic makes strand directionality visually concrete: the leading strand is synthesized continuously, while the lagging strand is synthesized discontinuously as short segments. Importantly, the arrows show that DNA synthesis proceeds 5′→3′ in both cases, because nucleotides are added only to a 3′-OH terminus. Source
Directionality as a Practical Skill in Diagrams and Sequences
When interpreting a strand:
Identify the 5’ end by looking for the terminal phosphate
Identify the 3’ end by locating the terminal -OH on the 3’ carbon
Remember that written sequences (letters like A, U, T, C, G) are presented 5’ to 3’, matching the direction of synthesis
Being able to label 5’ and 3’ ends helps you track which end is extending and prevents common errors such as placing an added nucleotide on the wrong side of a strand.
FAQ
This convention matches the direction the new strand is chemically extended (addition at the 3’ end).
It also standardises communication across biology (databases, primers, and published sequences), preventing ambiguity when comparing strands.
Look for the “odd group out” at each end of the backbone:
5’ end: terminal phosphate
3’ end: terminal OH on the sugar
If the diagram shows only the backbone, the 3’ end is the one that could plausibly be extended by adding another nucleotide.
Synthesis cannot continue because there is no 3’ OH to form the next phosphodiester bond.
This principle is used in laboratory methods that intentionally terminate chain growth by using nucleotides modified at the 3’ position.
Yes. The sugar differs (ribose instead of deoxyribose), but the key requirement—a free 3’ OH to extend the chain—remains the same.
So RNA strands are also synthesised by adding nucleotides to the 3’ end.
Changes are reported relative to a defined orientation. If you reverse the direction, positions and identities can be misread.
Keeping a consistent 5’ to 3’ frame helps you correctly locate where a base was added, removed, or substituted when comparing two sequences.
Practice Questions
State which end (5’ or 3’) of a nucleic acid strand is extended during synthesis and name the functional group required at that end. (2 marks)
1 mark: Extension occurs at the 3’ end.
1 mark: Requires a free 3’ hydroxyl (OH) group.
Describe how the chemical difference between the 5’ and 3’ ends of a nucleic acid strand explains why nucleotides are added only to the 3’ end during synthesis. (5 marks)
1 mark: 5’ and 3’ refer to the numbered carbons on the pentose sugar.
1 mark: The 5’ end has a terminal phosphate group (or identifies phosphate at 5’ carbon).
1 mark: The 3’ end has a terminal hydroxyl (OH) group (or identifies OH at 3’ carbon).
1 mark: Phosphodiester bonds form between the 3’ OH of the growing strand and the phosphate of the incoming nucleotide.
1 mark: Therefore elongation proceeds 5’ to 3’ because addition must occur where a free 3’ OH is available.

