AP Syllabus focus:
‘Describe how each nucleotide contains a five-carbon sugar, a phosphate group, and a nitrogenous base.’
Nucleic acids are built from repeating chemical subunits. Understanding the shared parts of a nucleotide—and how those parts vary—explains how DNA and RNA can store information while remaining chemically stable in cells.
Core Idea: What a Nucleotide Is
Nucleic acids are polymers, and their monomers are nucleotides. Every nucleotide has the same three-part plan: a five-carbon sugar, a phosphate group, and a nitrogenous base.
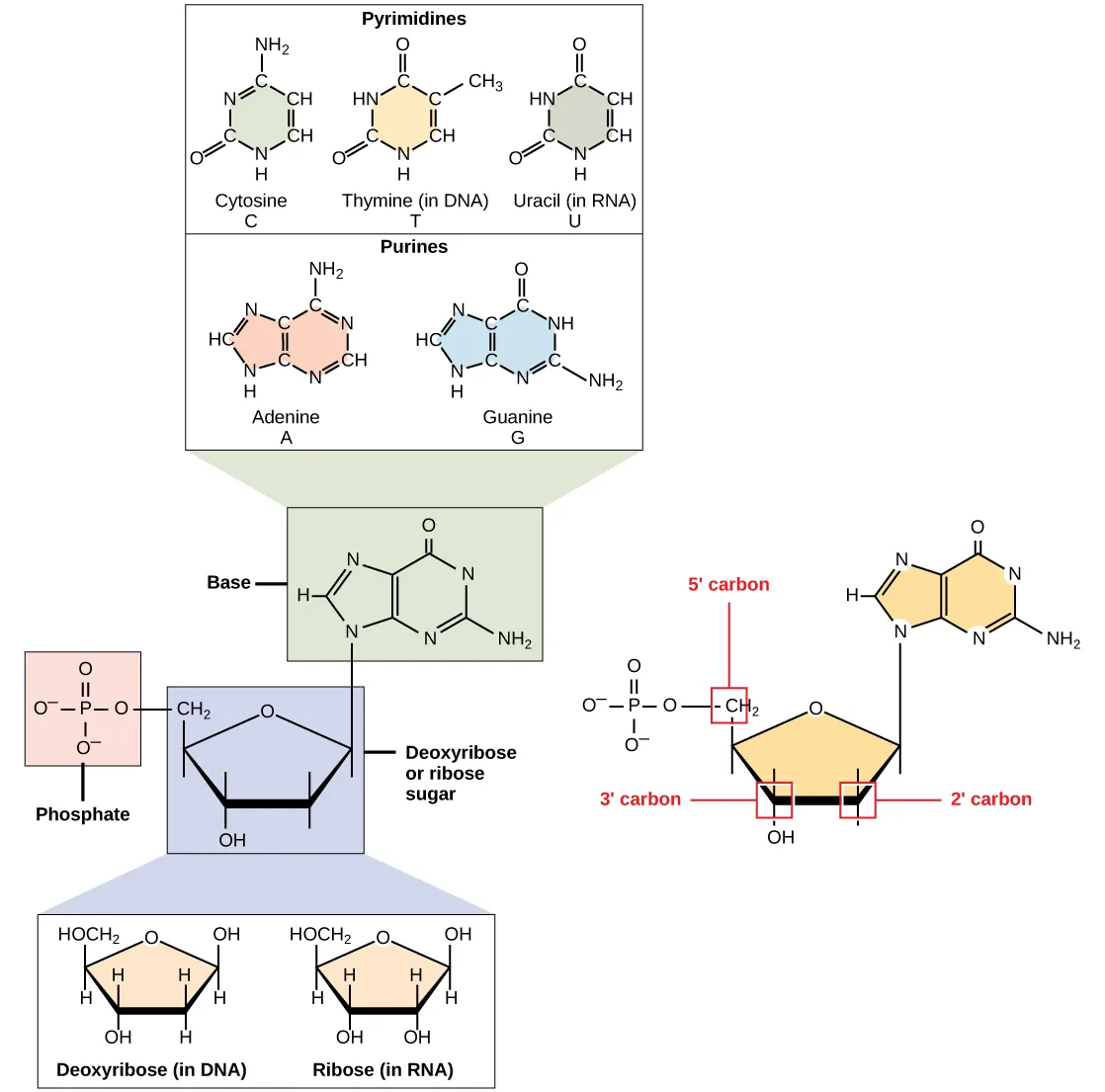
This diagram summarizes the shared nucleotide “architecture”: a pentose sugar with a nitrogenous base attached and one or more phosphate groups. It also visually distinguishes purines (two fused rings) from pyrimidines (single ring), linking base structure to base identity. Use it to anchor the idea that the sugar–phosphate portion is repetitive while the base is the variable information-bearing part. Source
Nucleotide: A nucleic-acid monomer composed of a five-carbon sugar, a phosphate group, and a nitrogenous base.
These three components give nucleotides two crucial properties:
a repeatable structural scaffold (sugar + phosphate)
a variable information-bearing part (nitrogenous base)
The Three Components in Detail
Five-Carbon Sugar (Pentose)
The five-carbon sugar is the central “hub” that connects the other two parts. Its carbons provide attachment points that allow nucleotides to be joined into long chains.
Key ideas for AP Biology:
The sugar is a pentose (five-carbon) molecule.
The sugar links to:
the nitrogenous base at one position
the phosphate group at another position
This consistent sugar structure helps produce a regular backbone in nucleic acids.
Phosphate Group
The phosphate group gives nucleotides important chemical behavior that affects nucleic acids as a whole.
Key ideas for AP Biology:
The phosphate is a functional group containing phosphorus and oxygen.
Phosphate groups are typically negatively charged in cells, which contributes to:
the overall negative charge of nucleic acids
interactions with water and positively charged ions/proteins
The phosphate group is part of what allows nucleotides to connect to one another during nucleic acid formation (without needing to memorise full reaction details here).
Phosphate group: A functional group containing phosphorus bonded to oxygen atoms, commonly contributing negative charge and participating in nucleotide connectivity.
Between definition blocks, remember that the phosphate is not the “information” part; it is part of the repeating structural framework that supports information storage.
Nitrogenous Base
The nitrogenous base is the variable component; differences in base identity allow nucleic acids to encode biological information.
Key ideas for AP Biology:
Bases are nitrogen-containing rings.
Bases fall into two structural categories:
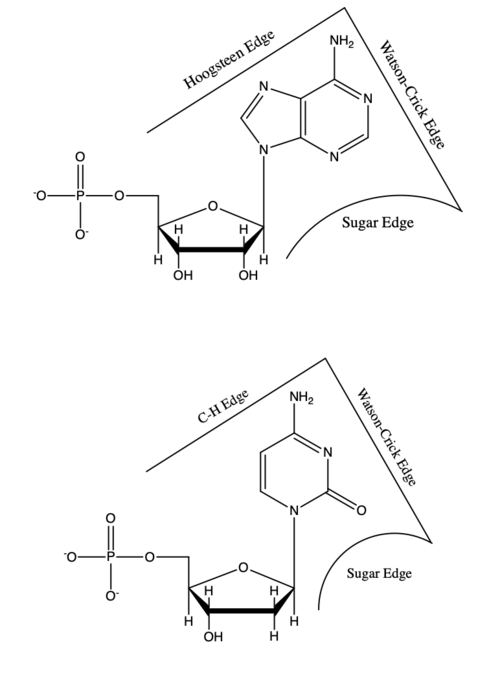
This figure compares purines and pyrimidines by highlighting their hydrogen-bonding “edges,” a structural feature that helps explain why specific base-pairing patterns are chemically favored. It reinforces the core classification: purines are larger (two-ring) bases and pyrimidines are smaller (one-ring) bases. Conceptually, it links base structure to how bases can interact in nucleic acids. Source
Purines: larger, two-ring structures (adenine, guanine)
Pyrimidines: smaller, one-ring structures (cytosine, thymine, uracil)
The order (sequence) of bases along a nucleic acid is what ultimately carries genetic instructions, while the sugar-phosphate structure provides consistency.
Nitrogenous base: A nitrogen-containing ring structure in a nucleotide whose identity can vary and thereby contribute to information storage in nucleic acids.
Related Terms Students Confuse
Nucleoside vs Nucleotide
A common point of confusion is whether phosphate is included.
Nucleoside: A molecule consisting of a five-carbon sugar bonded to a nitrogenous base, without a phosphate group.
A nucleotide = nucleoside + phosphate group. This distinction matters because adding phosphate changes both naming and chemical properties (especially charge and connectivity potential).
How Structure Supports Function (Syllabus-Aligned)
The syllabus emphasis is structural: each nucleotide contains:
a five-carbon sugar (central connector)
a phosphate group (part of the repeating framework and chemical reactivity)
a nitrogenous base (variable component enabling information storage)
Because every nucleotide shares the same overall architecture, cells can assemble long nucleic acid polymers with a uniform backbone while still achieving enormous variation through the sequence of bases.
FAQ
At physiological pH, phosphate oxygens tend to lose protons.
This creates one or more negative charges that are stabilised by resonance, making phosphate strongly polar and reactive in aqueous environments.
Not always.
Cells also contain nucleoside di- and triphosphates (two or three phosphates), which are important in metabolism and nucleic acid synthesis.
They differ in ring size, nitrogen placement, and potential hydrogen-bonding patterns.
These differences influence how bases pair and how enzymes recognise specific nucleotides.
Yes.
Some nucleic acids contain modified bases (for example, methylated bases), which can affect stability, gene regulation, or recognition by proteins.
Enzymes typically recognise a combination of features:
base shape and hydrogen-bond donors/acceptors
sugar orientation
number/position of phosphates
This molecular fit supports specificity in cellular reactions involving nucleotides.
Practice Questions
State the three components found in every nucleotide. (2 marks)
Five-carbon (pentose) sugar (1)
Phosphate group (1)
Nitrogenous base (1)
(Max 2 marks)
Explain how nucleotide structure allows nucleic acids to have both a consistent framework and variable information content. (5 marks)
Identifies sugar + phosphate as the consistent/repeating structural parts (1)
Identifies the nitrogenous base as the variable component (1)
Explains that different bases (e.g., purines vs pyrimidines, or different base identities) create sequence variation (1)
Links sequence variation to information storage/encoding (1)
Describes that the consistent sugar-phosphate framework supports formation of long polymers/overall structural stability (1)

