IB Syllabus focus:
‘Assess dissolved oxygen, pH, temperature, turbidity, nutrients, metals and TSS. Calculate BOD (mg O₂ L⁻¹ over five days) and use water quality indices to summarise contamination.’
Measuring water quality is essential for monitoring ecosystem health and human water use. Scientists apply abiotic tests, indices, and biological oxygen demand (BOD) to detect and summarise contamination.
Abiotic Tests of Water Quality
Abiotic (non-living) factors provide measurable data about the chemical and physical condition of water. These tests identify pollution, habitat suitability, and long-term changes.
Key Abiotic Parameters
Dissolved oxygen (DO): Determines oxygen available for aquatic organisms. Low DO often indicates pollution or eutrophication.
pH: Reflects acidity or alkalinity. Neutral pH (7) supports biodiversity; deviations stress organisms.
Temperature: Influences solubility of oxygen and metabolic rates of organisms. Higher temperatures lower DO and stress cold-water species.
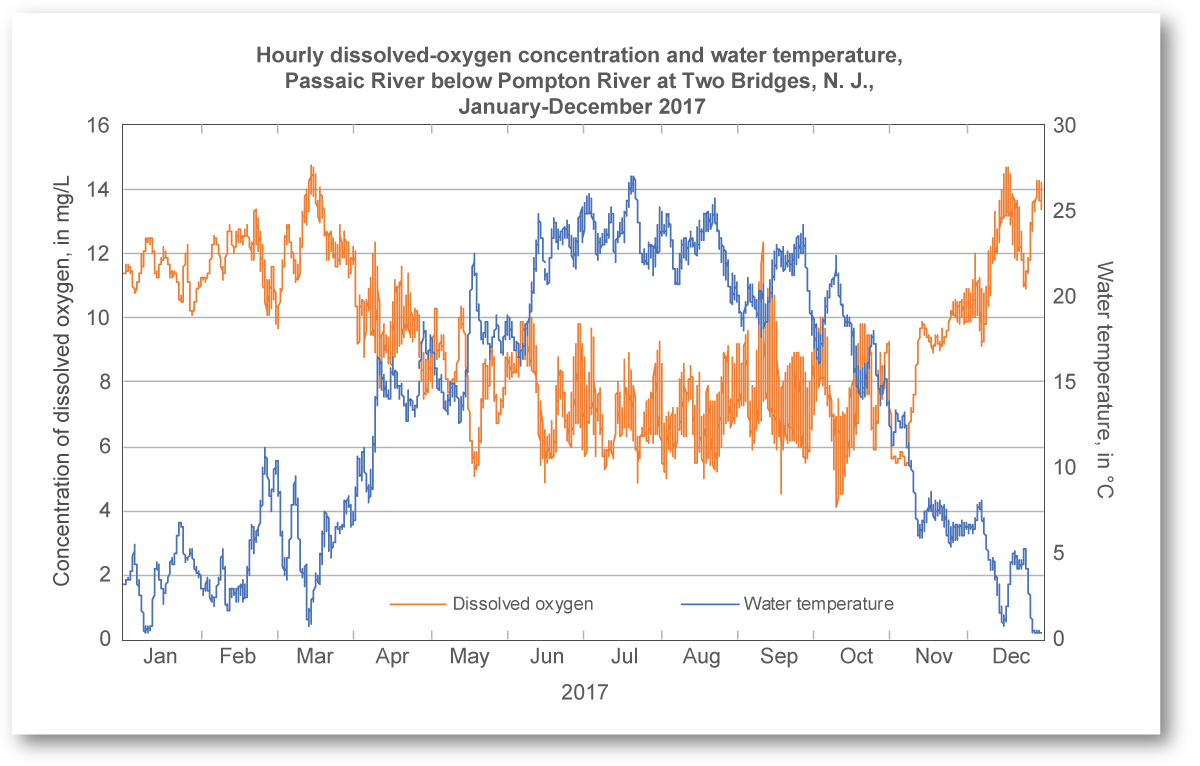
Graph showing hourly dissolved oxygen (mg L⁻¹) and water temperature (°C) through a year, illustrating the inverse relationship central to DO testing and interpretation. Site- and year-specific details are included, but the pedagogical focus is the trend, not the location. Source.
Turbidity: Measures suspended particles, affecting light penetration and photosynthesis. High turbidity reduces primary production and can clog gills.

Laboratory turbidimeters used for nephelometric turbidity measurements, a core abiotic test for water quality. The image focuses on the instruments and cuvettes relevant to IB-level methods. Brand/model details are present but not required by the syllabus. Source.
Nutrients (nitrates and phosphates): Excess levels trigger eutrophication.
Metals (e.g., lead, mercury, cadmium): Toxic even at low concentrations, bioaccumulate through food chains.
Total Suspended Solids (TSS): Solid matter carried in water, impacting clarity, sedimentation, and pollutant transport.
Dissolved Oxygen (DO): The concentration of oxygen gas (O₂) present in water, measured in mg L⁻¹, vital for aquatic life survival.
After DO, another crucial factor is pH, which influences nutrient solubility and toxicity of metals.
pH: A logarithmic scale from 0–14 measuring hydrogen ion concentration, where 7 is neutral, values <7 are acidic, and >7 are alkaline.
Importance of Abiotic Testing
Establishes baseline environmental conditions.
Detects early signs of contamination.
Guides water management decisions.
Supports compliance with environmental legislation.
Biological Oxygen Demand (BOD)
BOD is a widely used indicator of organic pollution, measuring how much oxygen microorganisms consume while decomposing organic matter in water.

Standard BOD test bottles used for five-day incubation at 20 °C, enabling calculation of BOD₅ from initial and final DO measurements. The photo was taken in a specific field context (constructed wetland), but the equipment shown is generic to BOD testing. Source.
Biological Oxygen Demand (BOD): The amount of dissolved oxygen required by aerobic microorganisms to break down organic material in water, usually measured over five days.
High BOD indicates heavy organic pollution, often linked to sewage or agricultural runoff, while low BOD reflects clean water.
BOD Measurement Process
Collect sealed water samples.
Measure initial DO concentration.
Incubate samples in darkness at 20°C for five days.
Measure final DO concentration.
BOD is calculated as the difference.
Biological Oxygen Demand (BOD) = DO_initial – DO_final
DO_initial = Dissolved oxygen at start (mg L⁻¹)
DO_final = Dissolved oxygen after 5 days (mg L⁻¹)
BOD provides insight into ecosystem stress and the ability of water bodies to support life. For example, a BOD above 8 mg L⁻¹ often signals severe organic pollution.
Water Quality Indices (WQI)
While individual tests provide raw data, water quality indices (WQIs) combine results into a single numerical value, simplifying interpretation and communication.
Water Quality Index (WQI): A numerical score derived from combining weighted measurements of abiotic water quality parameters, representing overall water condition.
Components of a WQI
Input parameters: DO, pH, temperature, turbidity, nutrients, metals, and TSS.
Weighting system: Each factor receives a significance weighting (e.g., DO higher than turbidity).
Calculation: Scores are combined mathematically to produce an index value.
Interpretation of WQI
High index value: Indicates clean, uncontaminated water suitable for drinking or ecosystems.
Moderate index value: Suggests potential concerns and reduced ecological health.
Low index value: Reflects poor water quality, high pollution, or unsuitable conditions for aquatic organisms.
Interconnections between Tests, BOD, and WQI
Abiotic tests, BOD, and WQI are not isolated. They complement one another:
Abiotic tests provide raw environmental data.
BOD highlights organic pollution load.
WQI translates complex data into an accessible summary.
Together, these measures allow governments, NGOs, and citizen scientists to monitor contamination, track changes, and support sustainable water management.
FAQ
DO levels vary with depth because photosynthesis, respiration, and decomposition occur at different intensities. Near the surface, photosynthesis by algae raises DO, while deeper layers often show depletion due to reduced light and higher decomposition rates.
Measuring DO across depths helps identify stratification, oxygen minimum zones, and potential risks such as hypoxic or anoxic conditions that threaten aquatic organisms.
Turbidity readings can be distorted by:
Presence of coloured dissolved substances, such as tannins.
Air bubbles or oils in the sample scattering light.
Particle size variation, as fine clay behaves differently from larger silt.
Regular calibration of turbidimeters and careful sampling are necessary to reduce these errors and ensure reliability of results.
The five-day period (BOD₅) is an international standard chosen because it approximates the time water takes to flow through many European rivers.
A temperature of 20°C is used because it provides consistent conditions for microbial activity without artificially accelerating or slowing decomposition. This standardisation allows results to be compared globally.
High concentrations of nitrates or phosphates can disproportionately lower WQI values because they signal a risk of eutrophication.
Weighting systems used in WQI calculations often assign greater importance to nutrients, meaning even small changes in their levels can shift the index significantly, reflecting their ecological impact.
TSS quantifies the mass of solid particles suspended in water, usually measured by filtering and weighing.
Turbidity measures light scattering by particles, providing a quick but indirect assessment.
Both are measured because turbidity is useful for rapid monitoring, while TSS gives more precise information about pollutant loads and sediment transport.
Practice Questions
Question 1 (2 marks):
Define Biological Oxygen Demand (BOD) and explain what a high BOD value indicates about water quality.
Mark scheme:
Definition of BOD: the amount of dissolved oxygen required by aerobic microorganisms to decompose organic material in water over a given period (1 mark).
High BOD indicates heavy organic pollution and reduced oxygen availability for aquatic life (1 mark).
Question 2 (5 marks):
Discuss how abiotic tests and the Water Quality Index (WQI) together provide a clearer understanding of water quality than using a single test in isolation.
Mark scheme:
Reference to abiotic tests measuring parameters such as DO, pH, temperature, turbidity, nutrients, metals, or TSS (1 mark).
Explanation that each abiotic test provides specific information about one aspect of water quality (1 mark).
Description of WQI combining results into a single weighted score or index value (1 mark).
Statement that WQI simplifies interpretation, allowing comparison between sites and communication to stakeholders (1 mark).
Link that together, abiotic tests and WQI offer both detailed data and an accessible overview, enabling more accurate monitoring and decision-making (1 mark).

