IB Syllabus focus:
‘The Sun emits across wavelengths; shorter UV has higher energy and greater biological risk (UVA, UVB, UVC).’
The Sun is Earth’s primary energy source, emitting a spectrum of radiation that sustains life and drives environmental systems. Understanding solar radiation and ultraviolet energy is essential in assessing ecological impacts, atmospheric processes, and human health.
The Solar Spectrum
Electromagnetic Radiation
The Sun emits electromagnetic radiation, which includes a wide range of wavelengths and frequencies. The solar spectrum can be divided into distinct regions:
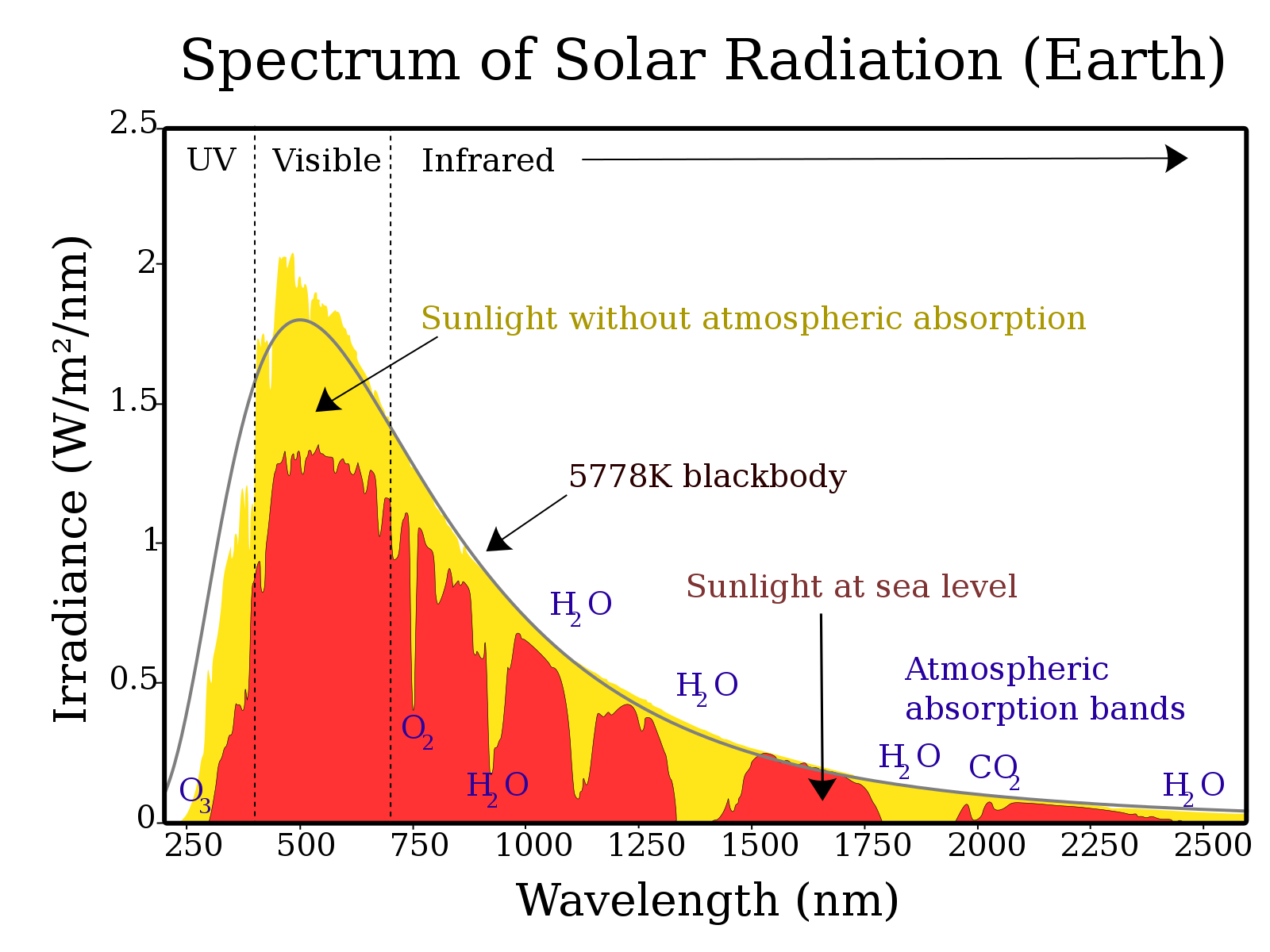
A labelled spectrum compares solar irradiance outside the atmosphere with that at sea level, clearly showing the ultraviolet, visible and infrared bands and the attenuation of shorter wavelengths by atmospheric gases. This visual reinforces that shorter UV wavelengths carry higher energy and that atmospheric filtering reduces UV at the surface. Source.
Ultraviolet (UV): 100–400 nanometres (nm)
Visible light: 400–700 nm
Infrared (IR): 700 nm–1 millimetre
Although visible light dominates solar energy reaching Earth, UV radiation carries significant biological and ecological importance.
Ultraviolet Radiation
Types of UV Radiation
Ultraviolet radiation is classified into three categories based on wavelength:
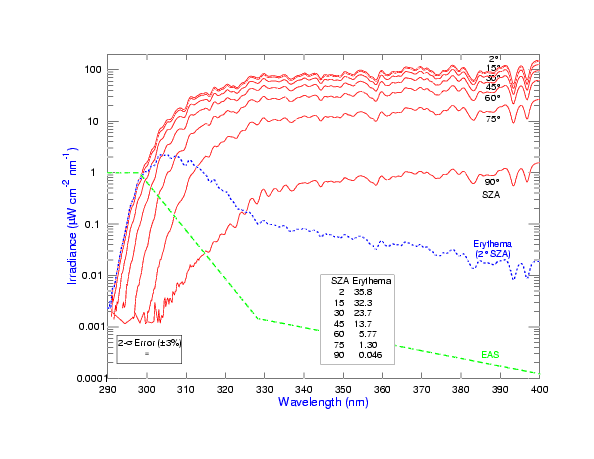
A NOAA graph of UV spectral irradiance at the surface illustrates how UV intensity varies across 290–400 nm and with solar zenith angle, reinforcing that shorter wavelengths (higher energy) dominate biological effects. The overlaid erythemal action spectrum reflects skin sensitivity—a small addition beyond the syllabus but helpful to contextualise biological risk. Source.
UVA (315–400 nm): Lowest energy UV, penetrates deep into skin and materials, associated with long-term biological effects like ageing.
UVB (280–315 nm): Medium energy, causes direct DNA damage, sunburn, and has more immediate biological consequences.
UVC (100–280 nm): Highest energy and most damaging, but normally absorbed completely by the atmosphere, especially by oxygen and ozone.
Ultraviolet radiation: Electromagnetic radiation from the Sun with wavelengths shorter than visible light but longer than X-rays, ranging from 100–400 nm.
Energy and Wavelength
Inverse Relationship
The energy of electromagnetic radiation is inversely proportional to wavelength. This means:
Shorter wavelengths (e.g., UVC) carry higher energy.
Longer wavelengths (e.g., UVA) carry lower energy.
Energy of a photon (E) = h × c / λ
E = Energy (Joules)
h = Planck’s constant (6.63 × 10⁻³⁴ Js)
c = Speed of light (3.0 × 10⁸ m/s)
λ = Wavelength (metres)
Thus, UVC photons are far more energetic and biologically hazardous than UVA photons.
Biological Risk of UV Radiation
Effects on Life
The syllabus specifies that shorter UV wavelengths carry greater biological risk. This is because:
UVC radiation has enough energy to break chemical bonds in DNA and proteins, causing lethal mutations.
UVB radiation damages cellular DNA and leads to skin cancers, cataracts, and impaired photosynthesis in plants.
UVA radiation contributes mainly to long-term damage such as premature ageing of skin and indirect DNA damage via free radicals.
Ecological Impacts
UV radiation influences not only humans but also ecosystems:
Reduces phytoplankton productivity, affecting marine food webs.
Damages leaf tissue in plants, lowering crop yields.
Alters animal behaviour and reproduction due to light-sensitive processes.
Atmospheric Interactions with UV
Absorption and Filtering
Earth’s atmosphere acts as a protective filter against UV:
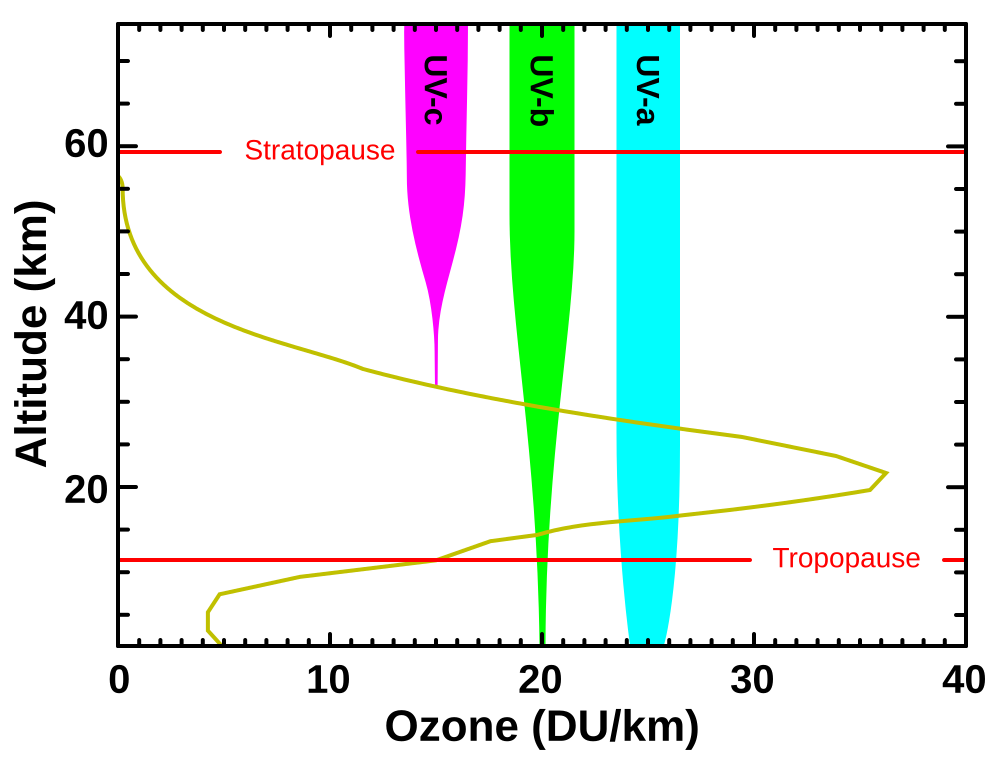
This diagram plots ozone concentration with altitude and indicates how UVC is fully blocked, UVB is mostly absorbed, and UVA largely transmits to the surface. It visually connects stratospheric ozone to the differential biological risks of the three UV bands highlighted in the syllabus. Source.
UVC radiation: Fully absorbed by oxygen and ozone in the stratosphere.
UVB radiation: Mostly absorbed, though some penetrates to Earth’s surface, especially when ozone concentrations decrease.
UVA radiation: Not significantly absorbed, reaching the surface almost completely.
This atmospheric filtering is essential for life, as without it, harmful UVC radiation would sterilise the planet’s surface.
The Importance of Ozone
Role of Stratospheric Ozone
Stratospheric ozone (O₃) is crucial for shielding Earth from UV radiation:
Absorbs all UVC radiation.
Absorbs most UVB radiation.
Allows UVA radiation to pass through largely unaffected.
Ozone layer: A region of the stratosphere with a relatively high concentration of ozone molecules, critical in absorbing harmful ultraviolet radiation from the Sun.
Without this natural shield, life on land would be impossible.
Human and Technological Relevance
Health Considerations
Human societies respond to UV exposure through:
Use of sunscreens to block or absorb UVB and UVA.
Designing UV filters in glasses and windows.
Establishing public health guidelines for outdoor activities.
Technological Applications
UV radiation is also harnessed for human use:
UVC lamps for sterilisation and disinfection.
UV-sensitive materials in medical imaging and scientific analysis.
Solar monitoring satellites to track UV levels and predict environmental impacts.
Summary of Key Points
The Sun emits a spectrum of electromagnetic radiation, including ultraviolet wavelengths.
UVA, UVB, and UVC differ in wavelength, energy, and biological impact.
Shorter wavelengths carry higher energy and greater biological risks.
Atmospheric gases, especially ozone, play a vital role in shielding Earth from harmful UV.
UV radiation has significant biological, ecological, and societal implications, making its study central to environmental science.
FAQ
The Sun emits energy across the entire electromagnetic spectrum due to the high temperatures of its surface. Visible light dominates, providing the primary energy for photosynthesis. Infrared radiation contributes to heating the atmosphere and surface, while ultraviolet makes up a small but significant fraction, influencing biological and atmospheric processes.
UV intensity is measured using radiometers or spectroradiometers positioned at ground stations. These instruments detect radiation at different wavelengths, often broken into UVA and UVB bands.
Satellite instruments also monitor UV penetration globally, helping scientists track variations caused by factors like ozone depletion, latitude, altitude, and seasonal changes.
UVC has the highest energy and can cause severe DNA damage, but it is completely absorbed by oxygen and ozone in the upper atmosphere before reaching Earth’s surface.
This filtering means organisms have not evolved defences against UVC in the same way they have for UVA or UVB, as exposure in natural conditions is virtually zero.
The amount of UVB depends on:
Ozone concentration: Lower levels allow more UVB through.
Altitude: Higher elevations receive greater UVB intensity.
Latitude: Equatorial regions experience stronger UVB due to direct solar angle.
Season and time of day: UVB is highest in summer and at midday.
UVA penetrates deeper into water and biological tissues than UVB. While it is less energetic, this depth of penetration means it can influence aquatic organisms such as phytoplankton.
It also generates reactive oxygen species in cells, leading to oxidative stress. This contributes more to long-term ecological and health impacts, such as reduced productivity in marine systems and accelerated skin ageing in humans.
Practice Questions
Question 1 (2 marks)
State two differences between UVA and UVC radiation in terms of wavelength and biological impact.
Mark scheme:
1 mark for identifying that UVA has longer wavelengths (315–400 nm) compared to UVC’s shorter wavelengths (100–280 nm).
1 mark for identifying that UVC carries higher energy and is more biologically damaging, whereas UVA has lower energy and mainly causes long-term effects such as skin ageing.
Question 2 (5 marks)
Explain how the atmosphere, particularly the ozone layer, influences the levels of UVA, UVB and UVC radiation reaching the Earth’s surface.
Mark scheme:
Up to 2 marks for describing that UVC is fully absorbed by oxygen and ozone in the stratosphere.
Up to 2 marks for describing that UVB is mostly absorbed, though some reaches the surface and can cause biological harm.
1 mark for stating that UVA is largely unaffected by atmospheric absorption and reaches the surface almost entirely.
Answers must link ozone’s protective role to the differential effects on the three UV bands. Partial credit available for partial explanations.

