IB Syllabus focus:
‘Depletion increases surface UVB; polar “holes” form each spring due to ODSs and seasonal polar stratospheric conditions.’
The effects of ozone depletion and the formation of polar ozone holes highlight the vulnerability of Earth’s stratospheric ozone to human-driven chemical pollutants and seasonal dynamics.
Ozone Depletion and Increased UVB
When the stratospheric ozone layer is reduced, more ultraviolet B (UVB) radiation reaches Earth’s surface. UVB is a short-wavelength, high-energy form of solar radiation that poses risks to biological systems.
Ecosystem impacts: Reduced phytoplankton photosynthesis, disruption of food webs, and damage to terrestrial plant tissues.
Human health impacts: Increased risks of skin cancers, cataracts, premature ageing, and weakened immune responses.
Material degradation: Faster breakdown of plastics, paints, and other materials exposed to sunlight.
Ozone Hole: A significant seasonal depletion of stratospheric ozone, most notably over the Antarctic, where ozone concentrations drop below 220 Dobson Units.
The increase in surface UVB due to ozone depletion is distinct from climate change, though both are global atmospheric issues.
Causes of Polar Ozone Holes
Role of Ozone-Depleting Substances (ODSs)
ODSs such as chlorofluorocarbons (CFCs), halons, and hydrochlorofluorocarbons (HCFCs) contain chlorine and bromine atoms that catalyse ozone destruction.
CFCs are stable in the troposphere but break down under strong UV radiation in the stratosphere, releasing chlorine.
One chlorine atom can destroy thousands of ozone molecules in a catalytic cycle.
Bromine, though less abundant, is even more effective at destroying ozone.
Polar Stratospheric Clouds (PSCs)
In winter, extremely low stratospheric temperatures form polar stratospheric clouds. These provide surfaces for chemical reactions that activate chlorine and bromine.

Polar stratospheric (nacreous) clouds above McMurdo Station, Antarctica, photographed during late winter when stratospheric temperatures are lowest. These clouds furnish surfaces for heterogeneous reactions that convert reservoir chlorine into active forms before sunlight returns. The striking iridescence is incidental to syllabus aims but helps identify PSCs in the field. Source.
Reservoir gases such as HCl and ClONO₂ are converted into active chlorine compounds.
With the return of sunlight in spring, these active compounds rapidly destroy ozone.
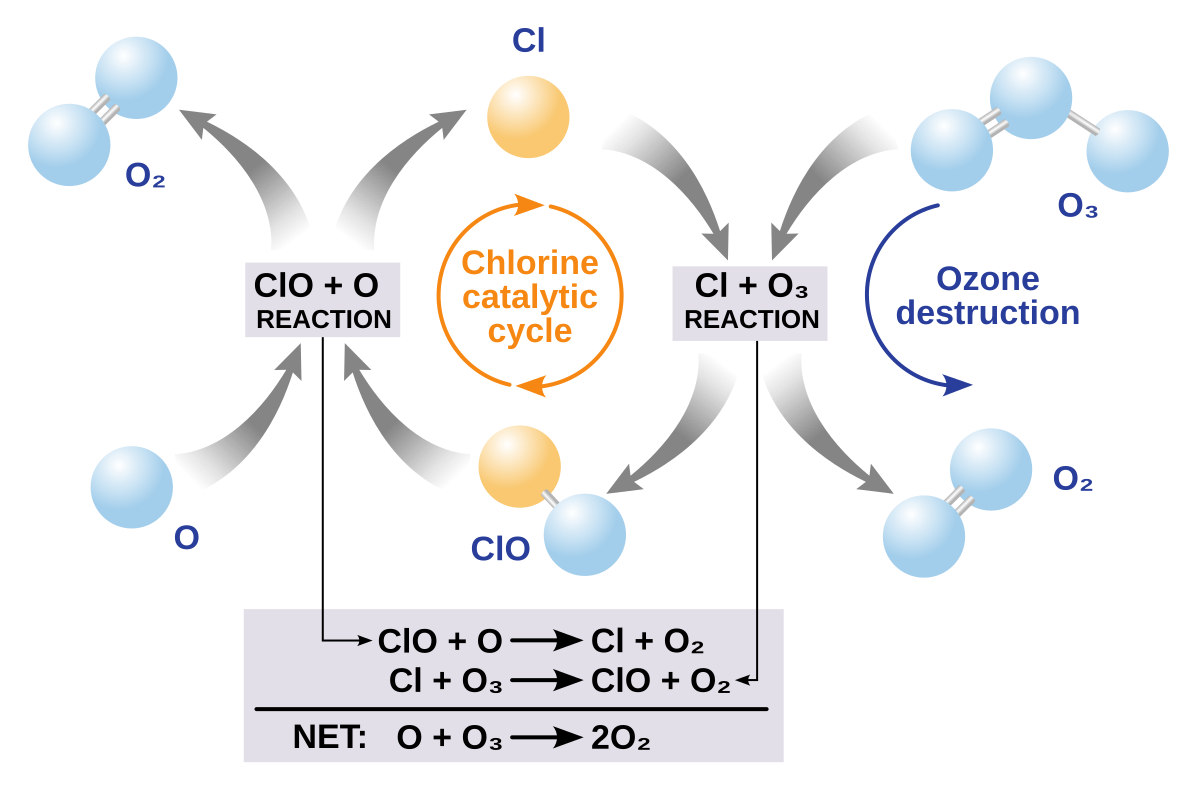
Schematic of the chlorine-catalysed ozone destruction cycle: Cl + O₃ → ClO + O₂; ClO + O → Cl + O₂; net conversion of odd oxygen to O₂. The diagram clarifies how sunlight maintains ClO and allows the catalyst to repeat, explaining rapid springtime losses after PSC processing. This includes mechanistic detail slightly beyond the syllabus but directly supports understanding of polar ozone holes. Source.
Polar Stratospheric Clouds (PSCs): Ice-crystal clouds forming in the polar stratosphere during winter that promote chemical reactions releasing ozone-depleting chlorine and bromine.
Antarctic vs Arctic Ozone Holes
Antarctic Ozone Hole
Most severe and persistent.
Caused by stable polar vortex, very cold stratospheric temperatures, and extensive PSC formation.
Appears each spring (September–November), with ozone levels dropping sharply.
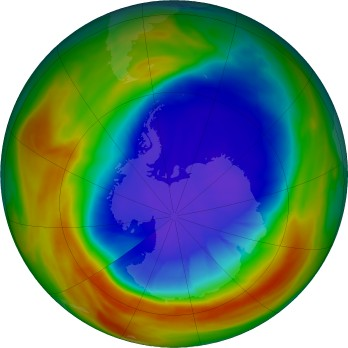
False-colour map of total column ozone over Antarctica showing the seasonal ozone hole (purple/blue) defined as regions under 220 Dobson Units. The scale bar links colours to DU values used in monitoring and reporting. This clean visual ties depletion to the springtime polar context that produces the hole. Source.
Arctic Ozone Depletion
Less severe and more variable.
Arctic stratosphere is warmer and less stable, with fewer PSCs.
Some years show significant thinning, but no consistent large-scale “hole” comparable to Antarctica.
Seasonal Dynamics
The seasonality of ozone holes is due to the interplay between darkness, cold temperatures, and sunlight:
Winter (polar night): Cold conditions form PSCs; chlorine and bromine reservoirs build up.
Spring (sunlight returns): UV radiation triggers chemical reactions, leading to rapid ozone destruction.
Summer: Temperatures rise, PSCs dissipate, and ozone begins to recover until the next cycle.
Scientific Monitoring and Measurement
Dobson Units (DU) measure total column ozone.
Normal ozone concentration: ~300 DU.
Ozone hole threshold: <220 DU.
Monitoring via satellites, ground-based spectrometers, and balloon-borne instruments provides evidence for long-term trends.
Dobson Unit (DU): A measure of ozone concentration, where 1 DU represents a layer of ozone 0.01 mm thick at standard temperature and pressure.
Ozone holes over Antarctica have been regularly documented since the 1980s, confirming the link between human emissions of ODSs and ozone depletion.
Consequences of Polar Ozone Holes
Biological Impacts
Higher UVB leads to DNA damage in plants and animals.
Marine ecosystems at risk due to reduced phytoplankton productivity, threatening fisheries and carbon cycling.
Agricultural crops exposed to increased UV stress show reduced yields.
Human Health
Increased skin cancers (basal cell carcinoma, squamous cell carcinoma, melanoma).
More cases of cataracts and other eye damage.
Immune system suppression reduces resistance to infectious diseases.
Environmental and Material Impacts
UV radiation damages synthetic materials, reducing their durability.
Degradation of paints, coatings, and polymers increases replacement costs.
Impacts on forest productivity and soil chemistry further stress ecosystems.
Distinction from Climate Change
It is essential to distinguish ozone depletion from global warming:
Ozone depletion: Primarily linked to ODSs and stratospheric chemistry.
Climate change: Driven by greenhouse gases (GHGs) and energy balance changes.
However, interactions exist: ozone loss can influence stratospheric circulation and climate patterns.
FAQ
Seasonal thinning refers to gradual reductions in ozone levels across many regions during certain times of the year.
The Antarctic ozone hole, however, is a dramatic and rapid drop below 220 Dobson Units confined mainly to Antarctic spring. It results from unique conditions: very cold stratospheric temperatures, stable circulation, and widespread polar stratospheric clouds.
As temperatures rise in late spring and summer, polar stratospheric clouds dissipate.
Without these clouds, active chlorine and bromine compounds revert to less reactive reservoir forms. This halts the rapid catalytic cycles destroying ozone, allowing levels to gradually rebuild until the next winter cycle.
Prediction involves monitoring:
Stratospheric temperatures during winter
Stability of the polar vortex
Extent and duration of polar stratospheric clouds
These factors determine how much chlorine and bromine are activated. A colder, longer winter usually leads to a deeper and larger ozone hole.
Volcanic eruptions inject aerosols into the stratosphere.
These aerosols increase surfaces for chemical reactions, enhancing ozone loss in regions already vulnerable. When combined with polar stratospheric cloud formation, eruptions can make seasonal ozone holes larger or longer-lasting.
Equatorial stratosphere is too warm for polar stratospheric clouds to form.
Without PSCs, reservoir chlorine compounds remain locked and inactive, preventing large-scale depletion. Thus, although sunlight is strong at the equator, the necessary chemical and thermal conditions for an ozone hole are missing.
Practice Questions
Question 1 (2 marks)
Identify two impacts of increased UVB radiation at Earth’s surface resulting from ozone depletion.
Mark scheme:
1 mark for each correct impact, up to 2 marks.
Acceptable answers include:
Increased incidence of skin cancers in humans (1)
Higher risk of cataracts and other eye damage (1)
Reduced phytoplankton productivity in marine ecosystems (1)
Lower crop yields due to plant tissue damage (1)
Faster degradation of synthetic materials such as plastics (1)
Question 2 (5 marks)
Explain why the Antarctic ozone hole is more severe than ozone depletion in the Arctic.
Mark scheme:
Award marks for the following points, up to 5 marks total:
Antarctic stratosphere is colder, leading to more extensive formation of polar stratospheric clouds (1).
PSCs provide surfaces for reactions that convert reservoir chlorine and bromine compounds into active forms (1).
The Antarctic polar vortex is stronger and more stable, isolating air masses and allowing depletion to intensify (1).
With the return of sunlight in spring, photochemical reactions destroy ozone rapidly (1).
The Arctic stratosphere is warmer and less stable, so PSC formation and depletion are less extensive and variable (1).
(Full marks require a clear comparison between Antarctic and Arctic conditions.)

