IB Syllabus focus:
‘Long-term ozone levels reflect a balance between formation and destruction processes.’
The ozone layer plays a critical role in shielding life on Earth from harmful ultraviolet radiation. Its long-term stability depends on a dynamic balance of formation and destruction processes in the stratosphere. Understanding this steady-state equilibrium provides insight into both natural atmospheric functioning and the threats posed by human activity.
The Nature of Ozone in the Stratosphere
What is Ozone?
Ozone (O₃): A molecule composed of three oxygen atoms, formed when molecular oxygen (O₂) absorbs high-energy ultraviolet radiation and splits, with free oxygen atoms combining with O₂.
Ozone is concentrated in the stratosphere, approximately 15–35 km above Earth’s surface, forming the ozone layer. Despite making up only a small fraction of atmospheric gases, it is vital in filtering out most ultraviolet B (UVB) and all ultraviolet C (UVC) radiation.
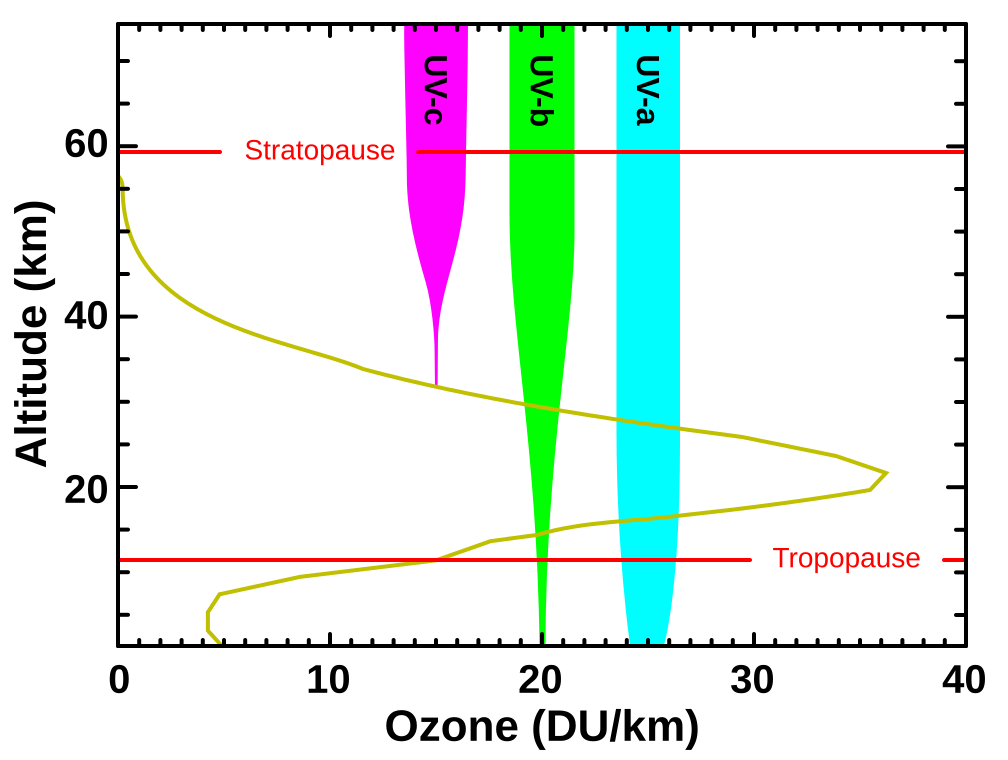
Graph of stratospheric ozone concentration and the penetration of ultraviolet radiation by type. It highlights that UVC is fully absorbed and most UVB is screened by ozone near 20–35 km altitude; UVA is included for context, which exceeds syllabus requirements but clarifies the spectrum. The figure supports the role of ozone in limiting biologically damaging UV at Earth’s surface. Source.
Formation of Ozone
Role of Ultraviolet Radiation
The Sun’s ultraviolet (UV) radiation initiates the creation of ozone through a series of photochemical reactions:
Step 1: Photodissociation of Oxygen Molecules
High-energy UVC radiation breaks apart molecular oxygen (O₂) into two individual oxygen atoms (O).
Step 2: Formation of Ozone
Each free oxygen atom (O) collides with an oxygen molecule (O₂), forming ozone (O₃).
These processes continuously generate ozone, particularly in the tropical stratosphere where UV radiation is strongest.
Destruction of Ozone
Natural Destruction Pathways
Ozone is inherently unstable and constantly destroyed through natural reactions:
Photodissociation of Ozone
Ultraviolet radiation (UVB) splits ozone (O₃) into O₂ and a free oxygen atom (O).
Recombination
Free oxygen atoms recombine with O₃, reducing ozone concentration.
Steady-State Equilibrium: A condition where the rates of formation and destruction of a substance are balanced, maintaining relatively stable concentrations over time.
This equilibrium ensures that although ozone molecules are continually formed and destroyed, the overall concentration remains relatively constant under natural conditions.
The Chapman Cycle
Explanation of the Process
The Chapman cycle, first described by Sydney Chapman in 1930, explains the chemical reactions governing ozone balance.
O₂ + UV → 2O
O + O₂ → O₃
O₃ + UV → O₂ + O
O + O₃ → 2O₂
This cycle underpins the steady-state equilibrium of ozone in the stratosphere.
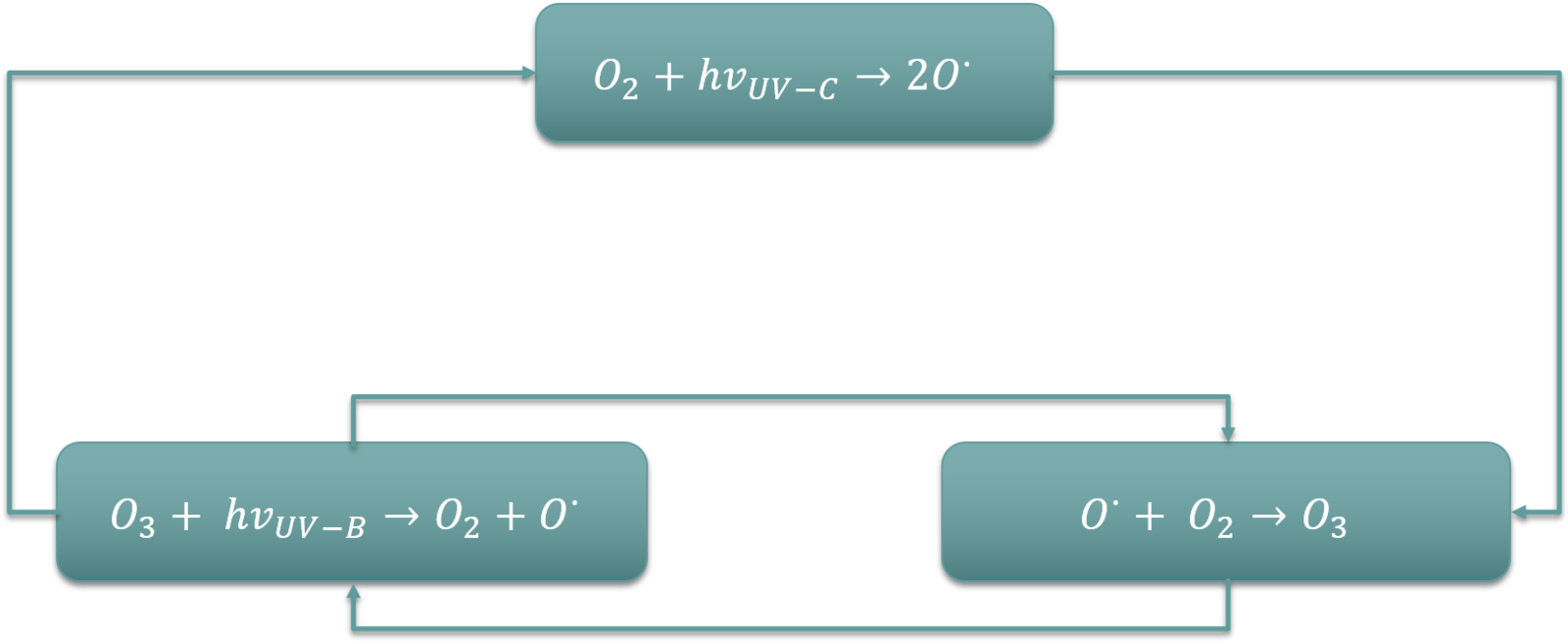
Diagram of the Chapman cycle showing photodissociation of O₂, ozone formation (O + O₂ + M → O₃ + M), and ozone loss (O₃ + hν → O₂ + O; O + O₃ → 2O₂). The schematic clarifies how opposing reactions maintain a dynamic steady-state ozone concentration in the stratosphere. Labels focus on processes required by the syllabus without extraneous detail. Source.
Factors Affecting Equilibrium
Natural Influences
Several natural processes influence the balance of ozone:
Solar activity: Variations in solar UV output alter ozone formation rates.
Stratospheric circulation: Winds redistribute ozone, affecting concentration at different latitudes.
Volcanic eruptions: Aerosols injected into the stratosphere can temporarily alter chemical reactions.
Anthropogenic Influences
While the steady-state equilibrium is naturally maintained, human activities can disrupt it:
Ozone-depleting substances (ODSs), such as chlorofluorocarbons (CFCs), release chlorine and bromine atoms that catalyse ozone destruction.
These reactions accelerate depletion far beyond natural rates, tipping the balance towards loss.
Importance of the Ozone Equilibrium
Biological Protection
The equilibrium sustains ozone concentrations sufficient to filter harmful UV radiation.
Disruption increases exposure, leading to higher risks of skin cancer, cataracts, and DNA damage in living organisms.
Climate Connections
Although distinct from the greenhouse effect, ozone levels influence stratospheric temperature and circulation patterns, indirectly affecting climate dynamics.
Key Points for IB Students
Ozone concentration reflects a dynamic equilibrium between formation and destruction.
Ultraviolet radiation drives both processes, making the Sun’s energy the central factor.
The Chapman cycle is the fundamental model of ozone balance.
Natural influences (solar cycles, atmospheric circulation) cause variations, but long-term stability is usually maintained.
Human activities, particularly the release of ODSs, disrupt equilibrium, leading to ozone depletion and associated environmental impacts.
FAQ
At higher altitudes, stronger ultraviolet (UV) radiation increases the rate of ozone formation but also accelerates destruction.
In the lower stratosphere, less UV radiation is present, so ozone is more stable but produced at slower rates. This vertical distribution creates the characteristic ozone concentration peak between 20–35 km.
The Chapman cycle predicts higher ozone concentrations than what is actually measured.
Additional catalytic cycles, involving trace gases such as nitrogen oxides and chlorine compounds, also contribute to ozone destruction. These reactions reduce ozone more efficiently than the Chapman cycle alone predicts.
Stratospheric temperature influences reaction rates.
At warmer temperatures, molecular collisions occur more frequently, increasing both formation and destruction processes.
At colder temperatures, particularly near the poles, destruction can dominate when other chemicals are present, disturbing equilibrium.
Seasonal shifts in sunlight change the availability of UV radiation.
During spring and summer, higher solar intensity increases ozone production.
In winter, limited sunlight slows formation, and pre-existing destruction processes can dominate, lowering concentrations.
Without O₂, the Chapman cycle cannot function.
O₂ acts as the starting material for ozone creation through photodissociation.
It also participates in recombination reactions that regenerate O₃.
Thus, continuous replenishment of O₂ through the biosphere is essential for sustaining stratospheric ozone balance.
Practice Questions
Question 1 (2 marks)
Explain what is meant by the term steady-state equilibrium in relation to stratospheric ozone.
Mark scheme:
1 mark for stating that steady-state equilibrium is when the rate of ozone formation equals the rate of ozone destruction.
1 mark for linking this to the result that ozone concentration remains relatively constant over time.
Question 2 (5 marks)
Using the Chapman cycle, describe how ozone is both formed and destroyed in the stratosphere, and explain how this maintains a steady-state equilibrium.
Mark scheme:
1 mark for describing that UV radiation splits O₂ molecules into individual oxygen atoms.
1 mark for describing that these oxygen atoms combine with O₂ to form ozone (O₃).
1 mark for explaining that ozone can be destroyed when UV radiation splits O₃ into O₂ and O.
1 mark for explaining that free oxygen atoms can react with ozone to form O₂.
1 mark for concluding that the balance between these formation and destruction processes maintains a steady-state concentration of ozone in the stratosphere.

