IB Syllabus focus:
‘Stratospheric ozone absorbs all UVC and most UVB, reducing harmful radiation at Earth’s surface.’
The ozone layer in the stratosphere plays a crucial role in sustaining life by filtering ultraviolet (UV) radiation from the Sun. Without this protection, DNA damage, ecosystem disruption, and increased health risks would severely threaten the biosphere. Understanding ozone’s function is essential for appreciating its role in maintaining Earth’s habitability.
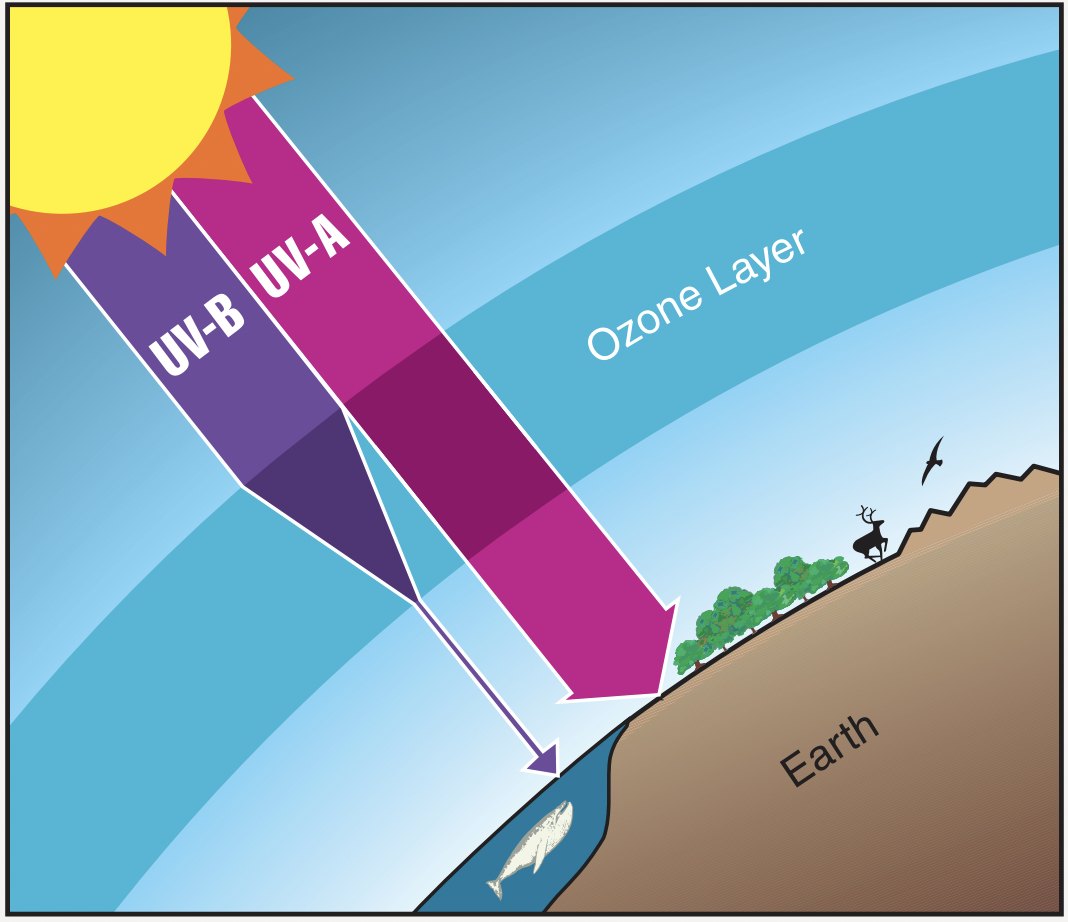
Illustration of the stratospheric ozone layer attenuating UVB while allowing most UVA to pass. The graphic emphasises ozone’s protective function over Earth’s surface in line with IB requirements. The figure focuses on UV shielding without delving into depletion chemistry beyond the syllabus need. Source.
The Solar Spectrum and UV Radiation
The Sun emits a broad spectrum of electromagnetic radiation. Of particular concern for living organisms is ultraviolet (UV) radiation, which has higher energy and shorter wavelengths than visible light. UV radiation is divided into three categories:
UVA (320–400 nm): Lowest energy, penetrates deeply into the skin, associated with ageing.
UVB (280–320 nm): Medium energy, biologically damaging, linked to sunburn, DNA mutations, and cataracts.
UVC (100–280 nm): Highest energy, extremely damaging to DNA, but absorbed almost entirely by the atmosphere.
Ultraviolet (UV) Radiation: A form of electromagnetic radiation with wavelengths shorter than visible light but longer than X-rays, classified into UVA, UVB, and UVC.
The Ozone Layer as a Protective Shield
The ozone layer is located in the stratosphere, approximately 15–35 km above Earth’s surface. It forms when solar radiation splits oxygen molecules (O₂), producing atomic oxygen that reacts with O₂ to form ozone (O₃).
Ozone Layer: A region of the stratosphere containing relatively high concentrations of ozone (O₃), responsible for absorbing harmful UV radiation.
Ozone Absorption of UV Radiation
UVC radiation is absorbed completely by stratospheric ozone and oxygen, preventing it from reaching Earth’s surface.
Most UVB radiation is absorbed by ozone, though a fraction reaches the ground, causing health risks.
UVA radiation passes through almost unaffected, but it is less biologically damaging compared to UVB and UVC.
This filtering process reduces harmful radiation, enabling life to flourish on Earth’s surface.
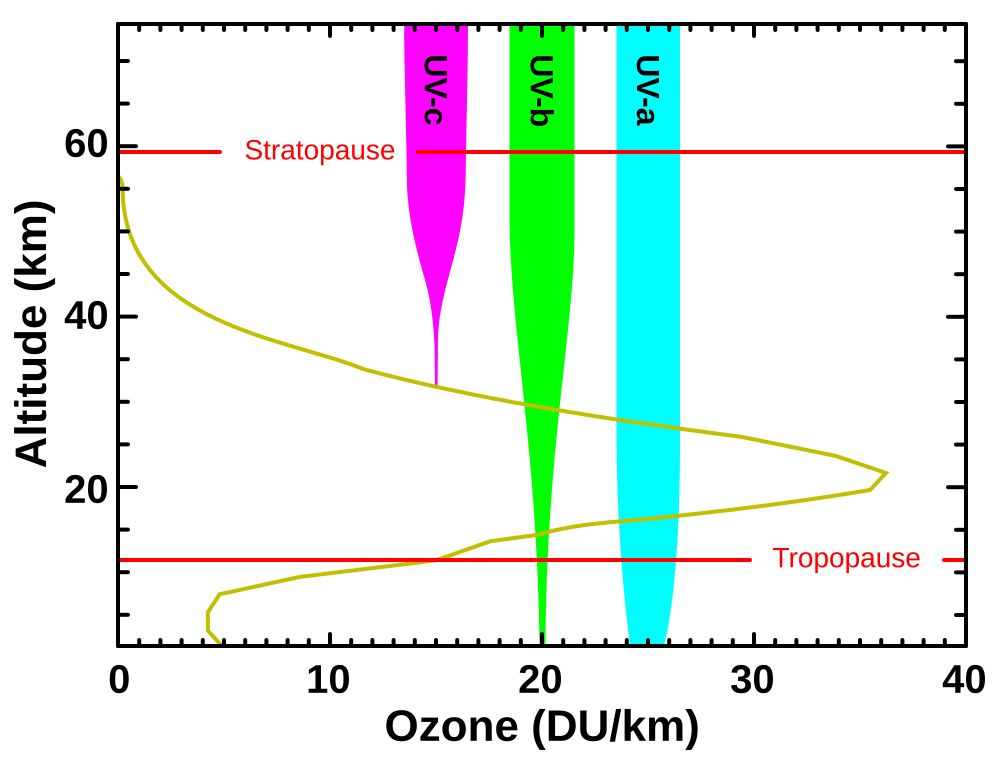
Diagram showing ozone concentration with altitude alongside the penetration depths of UVA, UVB, and UVC. It illustrates that UVC is fully blocked and most UVB is absorbed by the ozone layer, while UVA largely reaches the surface. Labels are minimal and aligned with IB ESS scope. Source.
The Photochemistry of Ozone
The protective capacity of the ozone layer depends on ongoing chemical reactions known as the ozone–oxygen cycle or Chapman cycle.
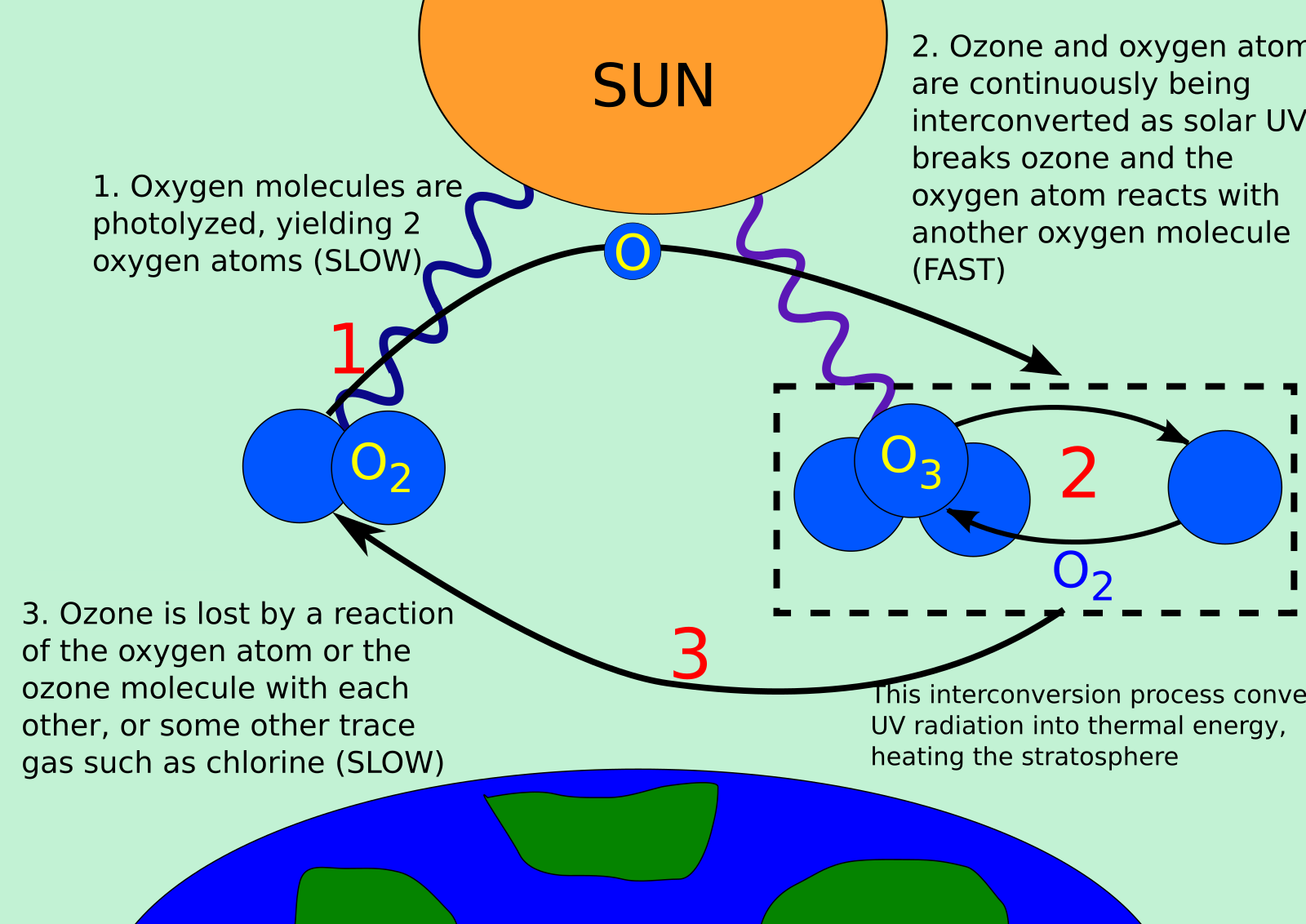
Schematic of the Chapman cycle: UV splits O₂ into O atoms, O combines with O₂ to form O₃, and O₃ absorbs UV and reverts to O₂ + O. This cycle both maintains stratospheric ozone and converts incoming UV into heat, supporting the layer’s shielding role. Labels are concise and aligned with IB ESS depth. Source.
Step 1: Solar UV radiation splits oxygen molecules (O₂) into two oxygen atoms.
Step 2: An oxygen atom reacts with O₂ to form ozone (O₃).
Step 3: Ozone absorbs UV radiation and splits into O₂ and an oxygen atom.
Step 4: The cycle repeats, maintaining a dynamic equilibrium.
This cycle ensures continuous absorption of UV radiation, especially UVB and UVC.
Biological Importance of Ozone Protection
Protection for Humans
Without ozone, intense UVB and UVC radiation would cause:
DNA mutations leading to skin cancers.
Eye damage, including cataracts.
Weakening of the immune system, making populations more vulnerable to diseases.
Protection for Ecosystems
Marine ecosystems: UV radiation damages phytoplankton, the base of aquatic food webs. Reduced photosynthesis here would disrupt global oxygen production and carbon cycling.
Terrestrial plants: Excess UV exposure impairs photosynthesis, stunts growth, and decreases crop yields.
Animals: UV radiation can damage skin and eyes across many species, reducing biodiversity and survival.
Key Characteristics of Stratospheric Ozone Shielding
Acts as a natural sunscreen for the planet.
Prevents UVC radiation from ever reaching Earth’s surface.
Absorbs around 90% of UVB radiation, reducing its harmful effects.
Protects the genetic integrity of living organisms by shielding DNA from mutation.
Maintains ecosystem productivity by safeguarding primary producers.
Human Dependence on Ozone Protection
Life on Earth evolved under the shield of stratospheric ozone. The presence of this layer allowed organisms to colonise land without lethal UV exposure. Today, human health, global agriculture, and fisheries all rely on its protective function.
Interconnection with Climate and Atmosphere
Although distinct from the greenhouse effect, ozone’s UV shielding interacts with atmospheric dynamics:
By absorbing UV, ozone warms the stratosphere, helping regulate atmospheric circulation.
This stratospheric heating influences weather patterns and energy distribution within Earth’s atmosphere.
Summary Points for Study
The ozone layer in the stratosphere is vital for life, shielding Earth from harmful solar UV radiation.
UVC is completely absorbed, most UVB is absorbed, while UVA passes through.
Ozone’s protective role prevents DNA damage, cancer, and ecosystem collapse.
Its function is maintained by the ozone–oxygen cycle, which constantly regenerates ozone molecules.
Human survival and ecosystem stability depend on the continued presence of this stratospheric shield.
FAQ
UVC has the shortest wavelength and highest energy of all UV types. This makes it extremely damaging to DNA, proteins, and cellular structures. If not absorbed by the ozone layer and oxygen in the upper atmosphere, UVC would cause immediate and severe biological harm, making surface life impossible. Its absence at ground level highlights the critical role of stratospheric ozone.
The ozone layer is not uniform; it varies by latitude and season.
Thickest at mid-latitudes.
Thinner at the equator and poles.
Seasonal fluctuations occur due to atmospheric circulation and sunlight intensity.
These variations influence the amount of UV radiation reaching different regions, with polar areas being especially vulnerable during spring when ozone levels can drop.
When ozone absorbs UV radiation, the energy is converted into heat, which warms the stratosphere. This process:
Maintains the thermal structure of the stratosphere.
Creates a temperature inversion that separates the stratosphere from the troposphere.
Influences global atmospheric circulation patterns.
The chemical properties of atmospheric gases determine absorption. Oxygen and ozone strongly absorb UVC, effectively blocking it. UVB is absorbed mostly, but not entirely, by ozone. By contrast, UVA is poorly absorbed by both oxygen and ozone, so it passes through largely unimpeded. Although less energetic, UVA still contributes to skin ageing and other health impacts.
The ozone layer’s formation around 600 million years ago coincided with the colonisation of land by plants and animals. Before its development, harmful UV levels would have prevented life from surviving outside water, which offered natural shielding. With ozone protection:
DNA stability increased.
Photosynthetic organisms could thrive on land.
Complex ecosystems emerged.
Thus, the ozone shield was crucial in shaping terrestrial biodiversity.
Practice Questions
Question 1 (2 marks)
State two ways in which the stratospheric ozone layer protects life on Earth from ultraviolet (UV) radiation.
Mark scheme:
1 mark for each valid point, up to 2 marks.
Acceptable answers include:
Absorbs all UVC radiation (1 mark).
Absorbs most UVB radiation (1 mark).
Reduces harmful radiation reaching Earth’s surface (1 mark).
Prevents DNA damage and mutation (1 mark).
Protects ecosystems and primary producers (1 mark).
(Any two for a maximum of 2 marks.)
Question 2 (5 marks)
Explain how the ozone–oxygen (Chapman) cycle maintains the ozone layer and contributes to reducing harmful ultraviolet radiation at Earth’s surface.
Mark scheme:
Describes photodissociation of oxygen molecules (O₂) into atomic oxygen by UV radiation (1 mark).
Describes formation of ozone (O₃) when atomic oxygen reacts with O₂ (1 mark).
Explains that ozone absorbs UV radiation and breaks down into O₂ and O (1 mark).
Explains that this cycle continuously regenerates ozone, maintaining its concentration (1 mark).
Links cycle to protection of life by reducing harmful UVB and UVC radiation at Earth’s surface (1 mark).
(Total: 5 marks)

