OCR Specification focus:
‘Use organisms safely and ethically to measure responses or physiological functions; use aseptic techniques and dissect organs safely.’
Working with organisms, microbiology and dissection are core practical competencies at A-Level. Investigations must be conducted responsibly, using ethical principles, aseptic procedure and accurate biological technique to obtain valid, reproducible data in laboratory or field settings.
Working with organisms
Students often carry out investigations using living organisms to collect biological response data such as respiration rate, taxis, kinesis or physiological change. Ethical practice prevents harm, minimises stress and maintains welfare when handling any organism. Ethical guidelines state that living organisms should be treated with respect, kept in suitable environmental conditions, and returned or disposed of appropriately after use.
Aseptic technique: A set of practices used to prevent contamination of cultures, equipment and the surrounding environment by microorganisms.
Organisms must be used only when necessary and when alternatives such as computer simulations cannot achieve the learning or investigative goal. Ethical considerations include the Replacement, Reduction and Refinement (3Rs), which limit suffering and unnecessary use of biological specimens. Good planning identifies variables, ensures valid controls and uses standardised methods so that results are reliable.
Experiments commonly measure responses in organisms such as woodlice, plant seedlings or invertebrates. Students must handle specimens gently, store them in appropriate habitats and avoid exposure to harmful conditions. Disposal or release must follow institutional and legal requirements.
Microbiology and aseptic procedure
Microbiology practicals develop skills in culturing, isolating and observing microorganisms. Microorganisms present significant contamination risks, so careful risk assessment and adherence to aseptic procedure are essential. Contamination can invalidate results and pose health hazards, so sterile technique underpins every stage of microbiological work.
Aseptic workspace and sterilisation
Work is normally performed near a Bunsen burner to create an updraft that reduces airborne contamination.
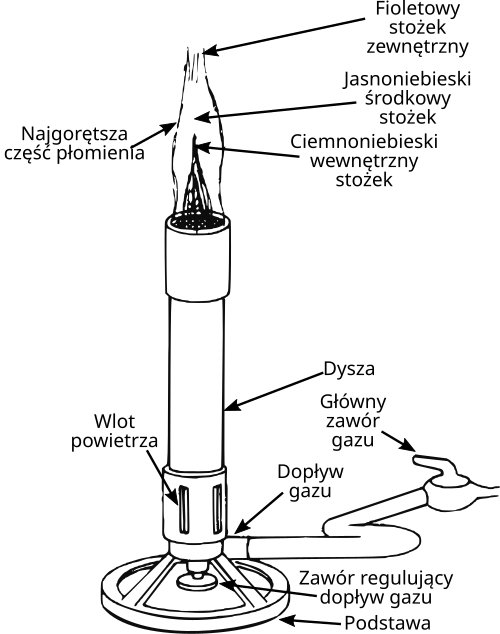
Labelled Bunsen burner diagram showing key parts used during aseptic technique. Understanding burner components supports correct, safe flame adjustment for sterilising tools and maintaining a clean field. The diagram includes extra structural detail (e.g., air hole/vent, collar) beyond the syllabus but directly aids safe operation. Source.
Surfaces are disinfected before and after work. Equipment such as inoculating loops is sterilised by heating until red-hot, and glassware may be sterilised in an autoclave. Cultures are opened only briefly, and lids are kept partially closed to prevent the entry of unwanted microorganisms.
Students preparing agar plates pour sterile molten agar into Petri dishes and allow it to set; plates are inoculated and then taped, but never fully sealed, to prevent anaerobic growth of pathogens. Incubation is normally at temperatures below 30 °C in schools to reduce the risk of culturing harmful human pathogens. After observation, cultures are destroyed, typically by autoclaving or chemical treatment, ensuring safe disposal.
Culture methods and observations
Microorganisms are transferred using:
• Streak plating to isolate single colonies
• Spread plating for an even lawn of growth
• Serial dilution to estimate population density
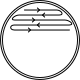
Quadrant streak plate technique illustrating progressive dilution across four sectors to yield isolated colonies. This diagram emphasises the need to flame and cool the loop between sectors to prevent carry-over. It visualises how isolation enables valid, reproducible microbiological data. Source.
Students record colony morphology, colour and distribution. Quantitative investigations might examine the effects of antibiotics or antiseptics on bacterial growth by measuring zones of inhibition, always using safe handling practices. Accurate labelling, sterile consumables and correct incubation conditions support valid, reproducible data.
Dissection skills and safety
Dissection develops understanding of anatomy and physiological function by allowing direct observation of tissues and organs. OCR A‑Level Biology requires students to dissect organs safely, using appropriate instruments such as scalpels, forceps and scissors.
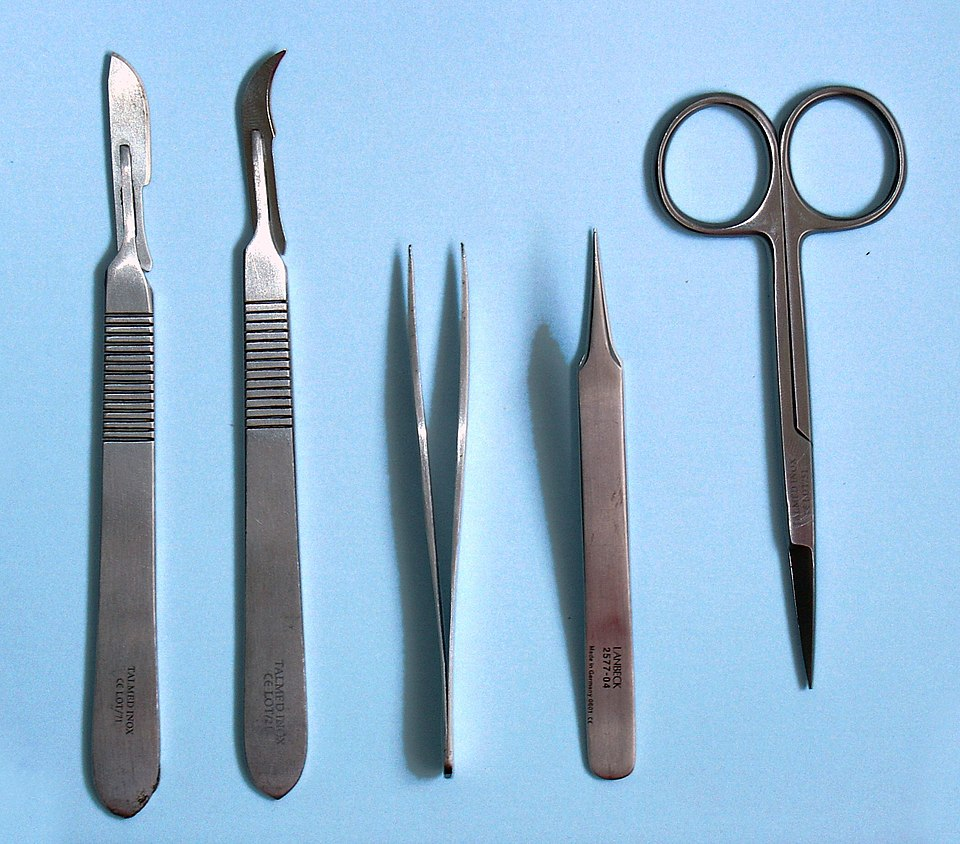
A standard dissection kit featuring scalpels, forceps and scissors as used in A-Level practicals. The image supports correct instrument recognition before handling and cleaning. No unnecessary technical clutter is included. Source.
Dissection: The methodical cutting and separation of tissues in an organism or organ to study anatomical structure.
Dissections must follow ethical sourcing rules, meaning specimens should come from approved suppliers and be stored hygienically. Students must wear eye protection, gloves and lab coats, keep blades sharp and cut away from the body and fingers. Tools should be disinfected after use and sharps disposed of in a designated container.
Technique and scientific recording
Dissection relies on steady handling, clear observation and accurate scientific drawings. Students identify structures such as vessels, valves, muscle layers or gas-exchange surfaces and relate these to function. Labelling must be neat, drawn in pencil, use single unbroken lines, and include magnification where appropriate. Moistening tissues with saline helps maintain structure during extended examination.
Risk management and good laboratory practice
All work with organisms, microorganisms or dissections requires a risk assessment identifying hazards, likelihood, severity and control measures. Common hazards include biological contamination, sharps injury and exposure to chemicals used in preparation or cleaning. Control measures include PPE, aseptic practice, proper training and strict waste management procedures.
Reliable investigations depend on:
• Clear identification of independent, dependent and control variables
• Sterile or controlled conditions to avoid confounding factors
• Detailed qualitative and quantitative records in a dated lab book
• Repeat trials to improve reliability and spot anomalies
By applying ethical handling, aseptic microbiological skills and safe dissection techniques, students meet the OCR requirement to use organisms competently while generating meaningful biological data.
FAQ
Schools use pre-checked, low-risk microbial species such as Bacillus or yeast and avoid known human pathogens. Plates are incubated below 30 degrees Celsius, which is too cool for many harmful bacteria to multiply efficiently.
Cultures are also destroyed immediately after use, usually by autoclaving or chemical disinfectants, preventing accidental release. Strict aseptic routines reduce contamination so that other microbes cannot gain a foothold and outgrow the intended culture.
Control plates help identify whether changes in bacterial growth are caused by the experimental variable or contamination.
For example, an uninoculated control plate should remain clear; any microbial growth on it indicates contamination. A plate with bacteria but no antibiotic acts as a comparison for assessing inhibition. Using controls makes data interpretation more confident and supports valid conclusions.
Stainless steel is durable, corrosion-resistant, and easy to sterilise in an autoclave or with disinfectant. This prevents rust and reduces the risk of contamination between specimens.
The material also keeps a fine cutting edge, improving precision when separating tissues. Stainless steel does not react with biological material, ensuring structures remain intact and uncontaminated for observation.
A high-quality drawing should be clear, biologically accurate and uncluttered. Key guidelines include:
• Use sharp pencil only
• Draw clean, unshaded lines
• Add neat, horizontal labels
• Include magnification if known
• Avoid crossing label lines
Scientific drawings focus on structure, not artistic shading, helping other scientists interpret the anatomy quickly and reliably.
Condensation can spread bacteria across the surface, causing merged colonies and making it impossible to isolate single colonies. This weakens the validity of experimental results.
To prevent this, plates are often incubated upside down so moisture stays on the lid. Cooler pouring temperatures for agar and leaving plates to dry before inoculation also help limit condensation.
Practice Questions
Question 1 (2 marks)
Describe two aseptic techniques that should be used when transferring bacteria onto an agar plate.
Question 1 (2 marks)
Award one mark for each correct aseptic technique described, up to a maximum of 2 marks:
• Flame the inoculating loop before use to sterilise it (1)
• Disinfect the workspace and keep the lid of the Petri dish partially closed to prevent contamination (1)
• Open containers for the shortest possible time (1)
• Work close to a Bunsen burner to reduce airborne contamination (1)
(Any two for 2 marks)
Question 2 (5 marks)
A student plans to investigate the effect of an antibiotic on the growth of bacteria using the streak plate method. Explain how the student should culture the bacteria safely and obtain valid, reproducible results in this investigation.
Question 2 (5 marks)
Award marks for the following valid points, up to a maximum of 5 marks:
• Use aseptic technique throughout, including flaming the loop and disinfecting the workspace, to prevent contamination (1)
• Correctly streak the plate to obtain isolated colonies and avoid cross-contamination between streaks (1)
• Tape, but do not fully seal, the Petri dish to prevent anaerobic growth of pathogens (1)
• Incubate at a safe temperature, normally below 30 degrees Celsius, to reduce the risk of culturing harmful microbes (1)
• Repeat the investigation or plate multiple replicates to ensure reliability and reproducibility of results (1)
• Clearly label plates and control variables (e.g., incubation time, agar type) for valid comparison (1)
(Any five for 5 marks)

