OCR Specification focus:
‘Explain oxygen binding and release, carbon dioxide transport via carbonic anhydrase and chloride shift; compare fetal versus adult haemoglobin and the Bohr effect.’
Gas transport in the blood ensures that oxygen reaches respiring tissues and carbon dioxide is efficiently removed. Haemoglobin’s structure and affinity changes underpin this dynamic physiological process.
Structure and Function of Haemoglobin
Haemoglobin (Hb) is a large, globular protein found in red blood cells (erythrocytes). Its primary role is to transport oxygen (O₂) from the lungs to the tissues and assist in carbon dioxide (CO₂) removal.
Haemoglobin: A conjugated protein made of four polypeptide chains (two α and two β in adults) each bound to a haem group containing iron (Fe²⁺).
Each haem group can bind one molecule of oxygen, allowing one haemoglobin molecule to carry up to four O₂ molecules. The iron ion in each haem group forms a reversible bond with oxygen, producing oxyhaemoglobin.
Oxygen Binding and Release
The binding of oxygen to haemoglobin is a cooperative process. When the first oxygen molecule binds, the haemoglobin molecule undergoes a conformational change, making it easier for subsequent oxygen molecules to bind — a phenomenon known as cooperative binding.
Conversely, as oxygen is released, haemoglobin’s affinity for oxygen decreases, promoting further oxygen unloading. This ensures efficient oxygen loading in the lungs and unloading in tissues.
Oxygen Dissociation Curve
The oxygen dissociation curve represents the relationship between the partial pressure of oxygen (pO₂) and the percentage saturation of haemoglobin.
The curve is sigmoidal (S-shaped) due to cooperative binding.
At low pO₂ (in respiring tissues), small decreases in pO₂ cause large decreases in saturation, promoting oxygen release.
At high pO₂ (in lungs), haemoglobin becomes rapidly saturated, ensuring efficient oxygen uptake.
Partial Pressure of Oxygen (pO₂): A measure of oxygen concentration, determining haemoglobin’s affinity for oxygen; expressed in kilopascals (kPa).
The Bohr Effect
The Bohr effect describes how increased carbon dioxide concentration or decreased pH reduces haemoglobin’s affinity for oxygen.
In actively respiring tissues, CO₂ dissolves in plasma forming carbonic acid, lowering pH. The hydrogen ions (H⁺) produced bind to haemoglobin, forming haemoglobinic acid (HHb), which causes oxygen to be released more readily.
This rightward shift of the oxygen dissociation curve allows haemoglobin to unload oxygen where it is most needed.
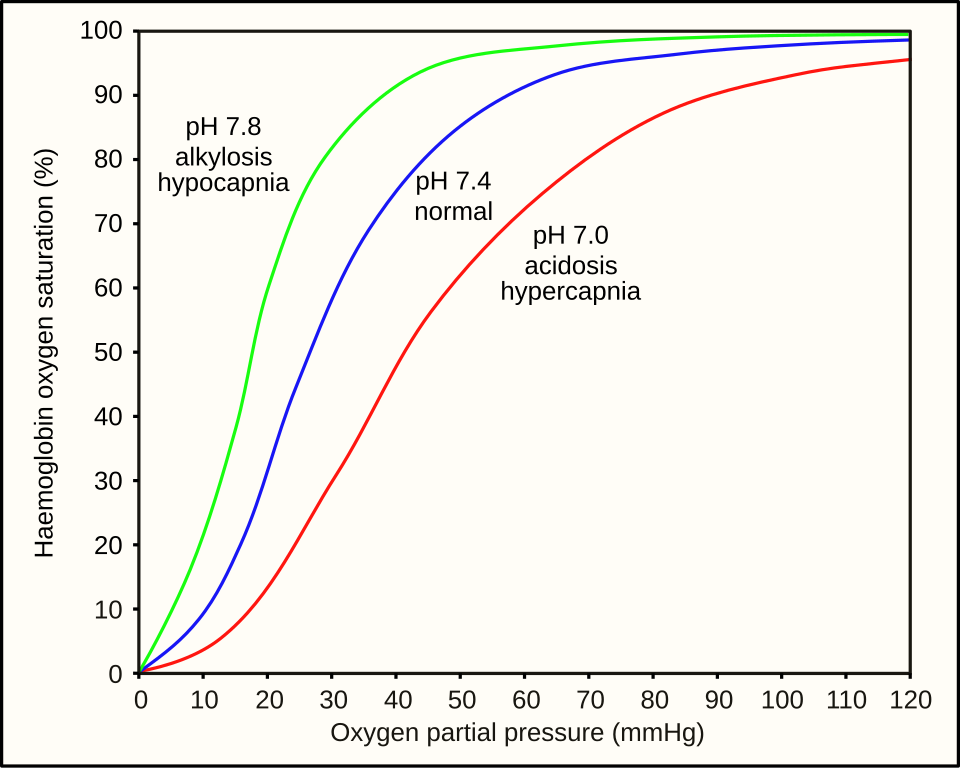
This diagram plots haemoglobin saturation against partial pressure of oxygen (pO₂) at different pH values. A lower pH shifts the curve to the right, reducing oxygen affinity and promoting release in respiring tissues (the Bohr effect). At higher pO₂ in the lungs and higher pH, the curve shifts left to support loading. Source.
Key points of the Bohr effect:
High CO₂ → low pH → decreased Hb affinity for O₂.
Promotes O₂ release in tissues.
Enhances O₂ loading in the lungs where CO₂ is exhaled and pH rises.
Carbon Dioxide Transport in Blood
Only a small proportion of CO₂ travels dissolved in plasma. Most is transported by conversion to hydrogen carbonate ions (HCO₃⁻) or bound to haemoglobin.
Pathways of CO₂ Transport:
About 5% dissolved directly in plasma.
Around 10–20% binds to haemoglobin to form carbaminohaemoglobin (HbCO₂).
Approximately 75–85% is converted into hydrogen carbonate ions (HCO₃⁻) within red blood cells, catalysed by carbonic anhydrase.
Carbonic Anhydrase: An enzyme found in erythrocytes that catalyses the reversible conversion of carbon dioxide and water into carbonic acid (H₂CO₃).
The carbonic acid formed then dissociates into H⁺ and HCO₃⁻ ions. The H⁺ ions combine with haemoglobin to form haemoglobinic acid, buffering blood pH, while HCO₃⁻ ions diffuse into plasma.
The Chloride Shift
To maintain electrical neutrality, when HCO₃⁻ ions leave the erythrocyte, chloride ions (Cl⁻) enter. This process is called the chloride shift.
Chloride Shift: The movement of chloride ions into erythrocytes as hydrogen carbonate ions move out, preserving electrical balance within red blood cells.
This exchange ensures that red blood cells can continue to transport CO₂ efficiently without disturbing ionic equilibrium.
Haemoglobin as a Buffer
Haemoglobin acts as a buffer, maintaining blood pH by binding free hydrogen ions produced during CO₂ transport. This prevents dangerous fluctuations in blood acidity that could disrupt enzyme activity or protein function.
Oxygen Transport in Different Haemoglobins
Adult vs. Fetal Haemoglobin
Fetal haemoglobin (HbF) differs structurally from adult haemoglobin (HbA), containing two γ-chains instead of β-chains. This difference confers a higher affinity for oxygen, ensuring efficient uptake of oxygen from the maternal blood across the placenta.
Comparison points:
HbF curve lies to the left of the adult curve.
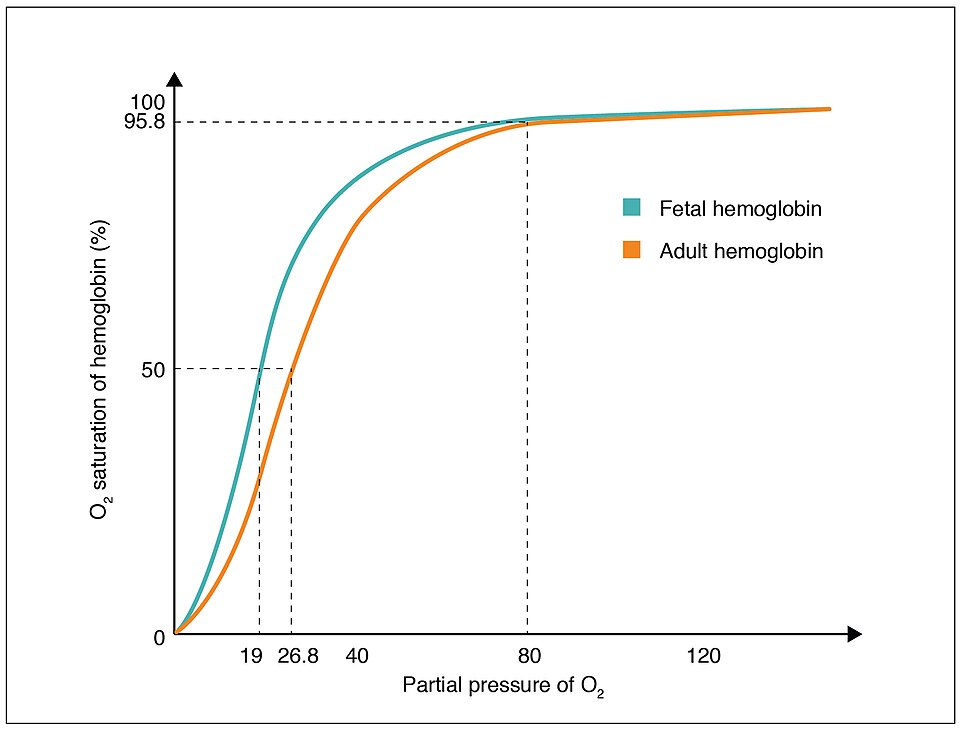
The fetal dissociation curve lies to the left of the adult curve, indicating higher oxygen affinity in fetal haemoglobin. This enables efficient transfer of O₂ from maternal HbA to fetal HbF at the placenta. Extra detail in the graphic (source credit) does not exceed syllabus needs. Source.
Enables oxygen transfer from mother (HbA) to fetus (HbF).
Essential for fetal development under relatively low oxygen tensions.
Affinity: The degree of chemical attraction between haemoglobin and oxygen; higher affinity means oxygen binds more readily and releases less easily.
Quantitative Aspects of Gas Transport
EQUATION
—-----------------------------------------------------------------
Oxygen Content (mL O₂/100 mL blood) = (1.34 × [Hb] × % saturation) + (0.003 × pO₂)
[Hb] = Haemoglobin concentration (g/100 mL blood)
pO₂ = Partial pressure of oxygen (kPa)
—-----------------------------------------------------------------
This relationship illustrates that the majority of oxygen is transported bound to haemoglobin rather than dissolved in plasma, highlighting haemoglobin’s crucial role in gas transport.
Integration of Oxygen and Carbon Dioxide Transport
The interdependence of oxygen and carbon dioxide transport is central to respiratory efficiency. As CO₂ levels rise in tissues:
The Bohr effect reduces oxygen affinity, promoting O₂ release.
Carbonic anhydrase catalyses CO₂ conversion to HCO₃⁻ for transport.
The chloride shift maintains ionic balance.
Haemoglobin buffers excess H⁺ to stabilise pH.
These combined mechanisms ensure optimal gas exchange, acid–base balance, and metabolic support throughout the organism.
FAQ
Increased temperature causes the oxygen dissociation curve to shift to the right, reducing haemoglobin’s affinity for oxygen. This is because higher temperatures weaken the bond between oxygen and the haem group, allowing oxygen to be released more readily in metabolically active tissues that generate heat.
Conversely, lower temperatures shift the curve to the left, increasing oxygen affinity and reducing release — a useful adaptation in tissues with low metabolic activity or in cold environments.
The S-shape reflects cooperative binding between haemoglobin subunits.
At low oxygen concentrations, haemoglobin binds oxygen slowly because the first binding event is difficult.
Once one oxygen molecule binds, the conformational change increases affinity, steepening the curve.
At high oxygen concentrations, the curve plateaus as haemoglobin becomes saturated.
This shape ensures oxygen is loaded efficiently in the lungs and released effectively in tissues.
Without haemoglobin buffering, free hydrogen ions from carbonic acid dissociation would accumulate in red blood cells and plasma, drastically lowering pH.
This acidosis would:
Denature key enzymes involved in metabolism.
Interfere with oxygen binding and release.
Reduce blood’s capacity to carry oxygen safely.
By binding hydrogen ions to form haemoglobinic acid, haemoglobin maintains pH near physiological levels, stabilising both respiration and enzyme function.
When bicarbonate ions (HCO₃⁻) leave the red blood cell, chloride ions (Cl⁻) enter to preserve the electrical balance.
This process prevents the cell from becoming overly positive, which would otherwise stop further bicarbonate export.
It also helps maintain osmotic stability, ensuring the cell neither swells nor shrinks as ion movements occur during high CO₂ transport activity in tissues.
During exercise, muscles produce more CO₂ and lactic acid, reducing blood pH. The Bohr effect causes haemoglobin’s affinity for oxygen to decrease, shifting the dissociation curve to the right.
This ensures that:
More oxygen is released where it is most needed.
Oxygen supply matches the high metabolic demand.
Haemoglobin efficiently reloads oxygen in the lungs where pH is higher and CO₂ levels are lower.
It is a critical adaptive mechanism for sustaining aerobic respiration during intense activity.
Practice Questions
Question 1 (2 marks)
Explain what is meant by the Bohr effect and describe its significance in active tissues.
Mark scheme:
1 mark for stating that increased carbon dioxide concentration or decreased pH reduces haemoglobin’s affinity for oxygen.
1 mark for stating that this causes oxygen to be released more readily in actively respiring tissues where CO₂ concentration is high.
Question 2 (5 marks)
Describe and explain how haemoglobin transports oxygen from the lungs to the tissues and how this process is affected by changes in carbon dioxide concentration.
Mark scheme:
1 mark for stating that haemoglobin binds oxygen in the lungs where the partial pressure of oxygen is high, forming oxyhaemoglobin.
1 mark for mentioning cooperative binding (each oxygen molecule binding makes it easier for the next to bind).
1 mark for explaining that at low partial pressures of oxygen (in tissues) haemoglobin releases oxygen.
1 mark for describing that increased carbon dioxide concentration or decreased pH causes a rightward shift in the oxygen dissociation curve (Bohr effect).
1 mark for explaining that this shift promotes oxygen release in respiring tissues where CO₂ concentration is high.

