OCR Specification focus:
‘Perform structured and unstructured calculations for redox titrations, including unfamiliar systems.’
Redox titration calculations rely on quantitative relationships between electron transfer, reacting species and titration stoichiometry. These notes outline key principles to approach unfamiliar systems confidently.
Understanding the Role of Redox Stoichiometry
Redox titration calculations are built upon the principle that electrons lost by the reducing agent equal electrons gained by the oxidising agent. This relationship underpins all quantitative analysis in redox titrations and allows chemists to calculate unknown concentrations, volumes, or reacting ratios. Redox titrations commonly involve species such as Fe²⁺/Fe³⁺, MnO₄⁻, and I₂/S₂O₃²⁻, but the OCR specification also requires the ability to handle unfamiliar combinations using systematic calculation approaches.
Oxidation Numbers and Electron Transfer
To interpret redox titration data, it is essential to identify changes in oxidation number, because these values reveal how many electrons are transferred in the reaction. Once the electron change is known, the stoichiometric relationship between the titrant and analyte becomes clear.
Oxidation Number: A numerical value representing the number of electrons an atom has gained, lost or shared in bonding relative to its elemental form.
Oxidation numbers enable consistent identification of electron-transfer processes across familiar and unfamiliar titration systems. This method ensures that even when species are not memorised, systematic redox handling remains possible.
Constructing Balanced Redox Equations for Calculations
Balanced redox equations form the foundation of every calculation. Students should construct full ionic equations using the half-equation method, ensuring mass and charge are conserved. This is essential because an incorrect equation leads directly to incorrect stoichiometric ratios in titration calculations.
The Half-Equation Method
This method separates oxidation and reduction processes, allowing electrons to be balanced before combining the two half-equations. It is especially helpful for titration systems involving polyatomic ions or multiple electron transfers.
Half-Equation: An equation showing either oxidation (electron loss) or reduction (electron gain) separately before combining to give an overall redox equation.
After balancing, the number of electrons transferred determines the ratio between the titrant and analyte, which is central to successful numerical work.
Essential Quantities in Redox Titration Calculations
Students will encounter key measurable quantities during titrations, all of which feed into final calculations. Volumes, concentrations, and reacting ratios interact through chemical stoichiometry and must be used with precision.
Moles, Concentration and Volume Relationships
At the core of calculations lies the relationship linking these three quantities. It must be applied after establishing the correct stoichiometric ratio from the redox equation.
Moles–Concentration Relationship (n = c × V)
n = Amount of substance in moles (mol)
c = Concentration of solution (mol dm⁻³)
V = Volume of solution (dm³)
This quantitative link is used repeatedly in redox titration calculations, making accurate unit conversions and volume readings essential throughout practical work.
A redox titration calculation starts with the titre: the volume of titrant delivered from the burette at the endpoint.
A standard titration setup showing a burette positioned above a conical flask to deliver titrant accurately. The measured titre volume obtained from this apparatus is used directly in redox titration calculations. Source
A clear stoichiometric ratio, based on the balanced redox equation, must then be applied to relate the moles of titrant to the moles of analyte.
Approaching Structured Redox Calculations
Structured calculations follow a predictable sequence, particularly in titrations such as Fe²⁺ versus MnO₄⁻ or I₂ versus S₂O₃²⁻. Even when systems differ, the mathematical logic remains constant, aligning with OCR expectations.
Step-Based Calculation Strategy
Use the following approach for both familiar and unfamiliar systems:
Identify the species undergoing oxidation and reduction.
Assign oxidation numbers and determine electrons transferred.
Write and balance both half-equations.
Combine to obtain the overall redox equation.
Determine the stoichiometric ratio for titration calculations.
Calculate moles of titrant using measured volume and concentration.
Use the mole ratio to determine moles of analyte.
Convert moles of analyte into required quantities (concentration, mass, or volume).
These steps ensure clear reasoning and minimise errors caused by skipping foundational chemical relationships.
Tackling Unstructured Calculations
The OCR specification requires students to perform unstructured calculations, meaning no step-by-step guidance is provided. Accordingly, students must independently choose relevant information, construct equations and identify necessary stoichiometric relationships.
Key Skills for Unstructured Questions
Unstructured problems often combine several concepts, so students must recognise which chemical and mathematical principles to apply:
Determine the overall purpose of the calculation, such as finding an unknown concentration.
Identify all measurable quantities present in the prompt.
Deduce which species are linked through redox electron transfer.
Construct and balance the redox equation without assistance.
Apply mole ratios: the essential link between titrant and analyte.
Convert final quantities into the units required by the question.
These calculations demand conceptual understanding rather than memorisation, particularly when encountering unfamiliar redox pairs.
Ensuring Accuracy in Redox Titration Calculations
Accuracy depends equally on chemical understanding and numerical precision. Misjudging oxidation numbers or misapplying mole ratios are frequent causes of error.
Record burette readings at eye level, using the correct point on the meniscus, and convert cm³ to dm³ before using concentration equations.
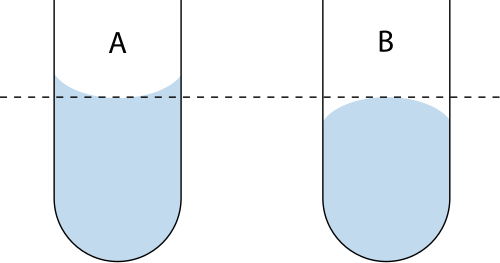
A diagram illustrating correct meniscus reading to avoid parallax error when measuring burette volumes. Accurate volume readings are essential for reliable redox titration calculations. Source
Common Pitfalls to Avoid
Students should be aware of typical mistakes to ensure reliable quantitative outcomes:
Incorrectly assigning oxidation numbers, especially in polyatomic ions.
Forgetting to balance electrons when combining half-equations.
Using unconverted volumes (cm³ instead of dm³).
Misreading burette titres or ignoring concordant values.
Applying the wrong stoichiometric ratio from the redox equation.
Confusing reducing and oxidising agents when determining electron flow.
Recognising these weaknesses supports more confident problem-solving in structured and unstructured titration questions aligned with the OCR A-Level specification.
FAQ
Concordant titres show that the titration technique is precise and repeatable. Using closely agreeing titres reduces random error when calculating mean titre values.
In redox calculations, small volume differences can significantly affect mole calculations, particularly when small sample volumes are used. Concordant results improve confidence in the final calculated concentration.
The number of electrons transferred by each reacting species determines the stoichiometric mole ratio used in calculations.
For example:
A one-electron change produces a 1:1 ratio
A five-electron change produces a 1:5 or 5:1 ratio
Ignoring electron transfer leads to incorrect mole relationships, even if volumes and concentrations are measured accurately.
Ionic equations show only the species that actually undergo electron transfer.
Spectator ions do not take part in the redox process and do not affect stoichiometry. Using ionic equations prevents incorrect inclusion of non-reacting species in mole ratio calculations.
Systematic errors arise from consistent inaccuracies rather than random variation.
Common causes include:
Incorrect concentration of the standard solution
Miscalibrated burettes or pipettes
Parallax error when reading volumes
Systematic errors affect all titres similarly and cannot be corrected by repeating the titration.
The analyte is the solution of unknown concentration, while the titrant has a known concentration.
Confusing these roles can result in:
Using the wrong volume in calculations
Applying the mole ratio incorrectly
Calculating the wrong concentration
Clear identification ensures calculations are logically structured and chemically valid.
Practice Questions
A student performs a redox titration in which acidified manganate(VII) ions react with iron(II) ions.
Explain why the balanced redox equation must be known before carrying out any calculations using the titre.
(2 marks)
Award marks as follows:
1 mark for stating that the balanced redox equation shows the ratio in which the reactants react
1 mark for linking this ratio to determining the correct mole relationship between Fe²⁺ and MnO₄⁻ (or oxidising and reducing agents)
In a redox titration, 25.0 cm³ of a solution containing Fe²⁺ ions is titrated with a solution of oxidising agent X.
The concentration of X is known.
The reaction between Fe²⁺ and X involves electron transfer.
Describe how you would use the titration data to calculate the concentration of Fe²⁺ ions in the original solution. You are not required to perform any calculations.
(5 marks)
Award marks as follows:
1 mark for identifying oxidation and reduction and determining the number of electrons transferred
1 mark for writing or using a balanced redox equation (or half-equations) to obtain the stoichiometric ratio
1 mark for stating that the moles of oxidising agent are calculated from its concentration and titre volume
1 mark for using the mole ratio to calculate the moles of Fe²⁺ ions
1 mark for converting moles of Fe²⁺ into concentration using the original solution volume
Maximum 5 marks.

