OCR Specification focus:
‘Apply electrode potential principles to storage cells; explain how fuel cells generate voltage from fuel and oxygen.’
Introduction
Storage cells and fuel cells are essential electrochemical technologies that apply principles of electrode potentials to produce electricity efficiently and reliably. Their behaviour depends on redox reactions.
Understanding Storage Cells
Storage cells, often called rechargeable cells, operate through redox reactions that can be reversed during recharging. Their behaviour is governed by standard electrode potentials, which determine the direction of electron flow and the maximum cell voltage.
Structure and Operation
A storage cell contains two electrodes with distinct electrode potentials. Electrons flow from the more negative electrode to the more positive electrode when connected in a circuit.
Negative electrode (anode during discharge): site of oxidation.
Positive electrode (cathode during discharge): site of reduction.
Electrolyte: allows ionic movement to maintain charge balance.
External circuit: allows electron transfer and electrical work.
During discharge, chemical energy converts to electrical energy; during charging, the process is driven in reverse using an external power source.
Electrode Potentials in Storage Cells
Electrode potentials determine both the feasibility and voltage of the cell reaction. A larger difference in standard electrode potentials results in a higher cell potential.
Cell Potential (E°cell) = E°(positive electrode) − E°(negative electrode)
E°(positive electrode) = Standard reduction potential of the cathode (V)
E°(negative electrode) = Standard reduction potential of the anode (V)
Storage cells are designed so that their electrode reactions are reversible and stable over many cycles. This depends on both the chemical composition and the physical structure of the electrodes.
Common Types of Storage Cells
Several rechargeable technologies exist, but their operation follows the same electrochemical principles.
Lead–Acid Cells
Used in automotive applications, these deliver high currents due to low internal resistance.
Positive electrode: PbO₂
Negative electrode: Pb
Electrolyte: aqueous H₂SO₄
The best-known storage cell is the lead–acid battery, where the cell reactions are reversible so the battery can be recharged.
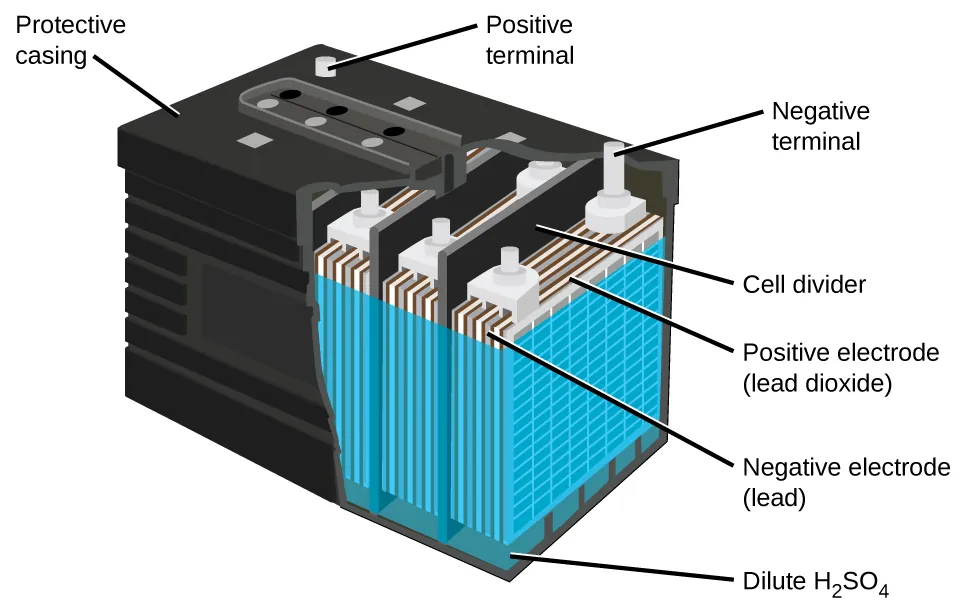
This cutaway shows a lead–acid battery cell with lead (negative plate), lead dioxide (positive plate), and dilute sulfuric acid as the electrolyte. It links the idea of a rechargeable storage cell to real battery construction and electrode polarity. Source
Lithium-Ion Cells
Widely used in portable electronics and electric vehicles due to high energy density.
Involves movement of Li⁺ ions between intercalated electrode materials.
Provide high voltage because of the large difference in electrode potentials.
Fuel Cells
Fuel cells generate electricity from the continuous supply of a fuel and an oxidant. Unlike storage cells, they do not store chemical energy internally but convert it directly as fuel is consumed.
Key Concept of Fuel Cells
Fuel cells rely on redox reactions involving a fuel (commonly hydrogen) and an oxidant (usually oxygen). They maintain a constant voltage as long as fuel and oxidant are supplied.
Fuel Cell: A device that generates electrical energy from the redox reaction between a continuously supplied fuel and an oxidant.
Fuel cells offer high efficiency and low pollutant output, making them important in low-emission energy systems.
Electrode Potentials in Fuel Cells
Electrode potentials determine the overall cell voltage. The hydrogen–oxygen fuel cell is a key example used within the OCR specification.
Anode (fuel electrode): hydrogen undergoes oxidation.
Cathode (oxygen electrode): oxygen undergoes reduction.
Electrons flow through an external circuit, while ions move through the electrolyte.
Hydrogen–Oxygen Fuel Cell
This fuel cell can operate in either acidic or alkaline electrolyte conditions.
In a hydrogen–oxygen fuel cell, hydrogen is oxidised at the anode and oxygen is reduced at the cathode, producing a potential difference as long as reactants are supplied.
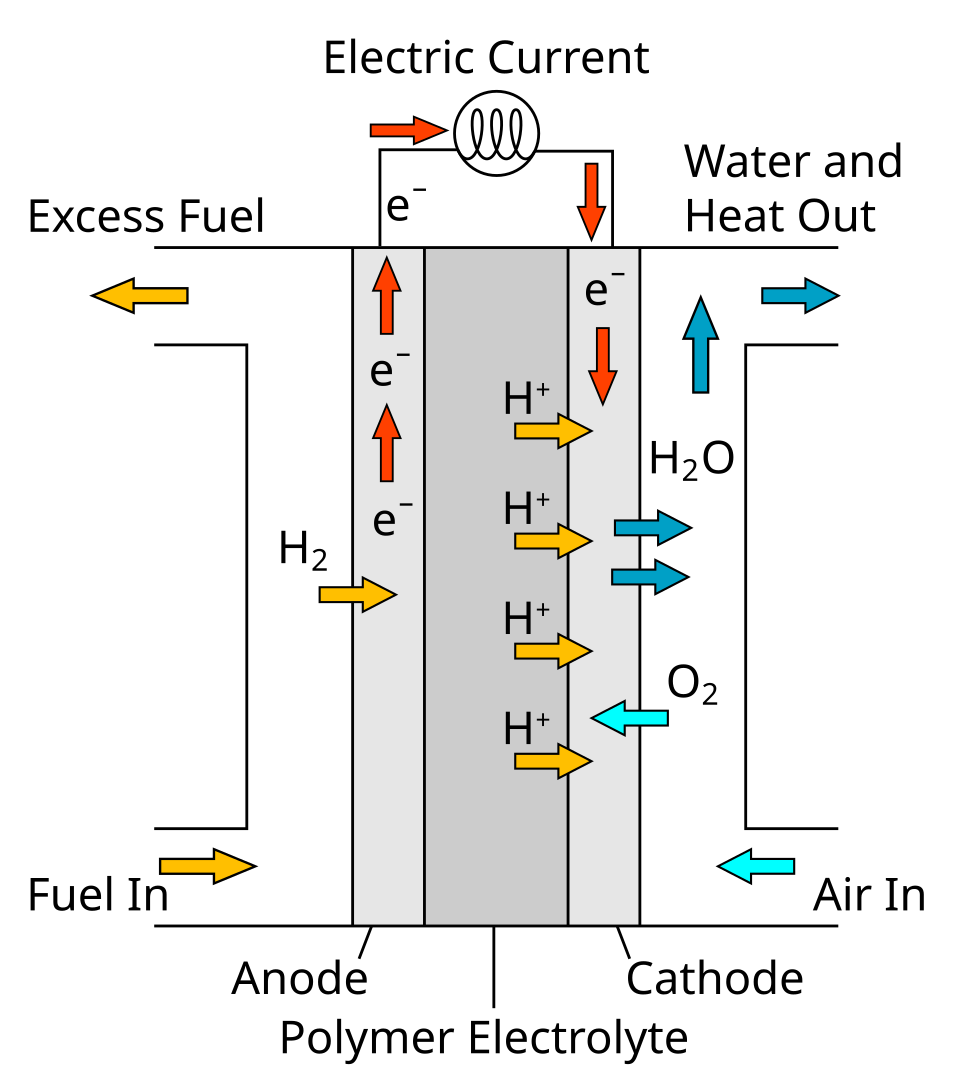
This schematic illustrates a hydrogen–oxygen fuel cell, showing electron flow through the external circuit and ion movement through the electrolyte. The polymer electrolyte membrane detail goes beyond the specification but clearly demonstrates how continuous fuel and oxygen supply generate electrical energy. Source
Acidic Conditions
Hydrogen is oxidised at the anode to form H⁺ ions.
Oxygen is reduced at the cathode to form water.
Alkaline Conditions
Hydrogen is oxidised to produce water and electrons.
Oxygen is reduced to form hydroxide ions.
Advantages of Fuel Cells
Fuel cells are valued for their efficiency and sustainability. Key advantages include:
Constant voltage output while fuel is supplied
High efficiency from direct electrochemical conversion
Low emissions when hydrogen is used
Useful in transport, stationary power, and portable applications
Comparing Storage Cells and Fuel Cells
Energy Source and Longevity
Storage cells store finite chemical energy internally; fuel cells generate energy continuously from an external fuel supply.
Reversibility
Storage cells are rechargeable; fuel cells are not recharged but refuelled.
Voltage Behaviour
Fuel cells provide a nearly constant voltage, whereas the voltage of storage cells decreases as they discharge.
Processes in Storage Cells and Fuel Cells
To clearly understand their operation, consider the key processes:
In Storage Cells
Oxidation occurs at the negative electrode during discharge.
Reduction occurs at the positive electrode during discharge.
Recharging reverses electron flow using an external power source.
In Fuel Cells
Fuel is continuously supplied to the anode.
Oxidant is supplied to the cathode.
Products are removed to maintain reaction efficiency.
Electrode potentials govern voltage output.
These principles allow both storage cells and fuel cells to function as reliable electrochemical energy sources guided by electrode potentials.
FAQ
Storage cells lose capacity over time because their electrode materials slowly degrade.
This can happen due to:
Physical changes such as cracking or shedding of electrode material
Side reactions that consume active substances
Incomplete reversal of reactions during recharging
These processes reduce the amount of material available for the main redox reactions, lowering the maximum charge the cell can store.
Fuel cells convert chemical energy directly into electrical energy rather than first producing heat.
This avoids large energy losses associated with:
Heat dissipation
Mechanical inefficiencies in moving parts
As a result, a greater proportion of the fuel’s energy is converted into useful electrical work.
Many fuel cells use catalysts at the electrodes that are easily poisoned by impurities.
Small amounts of substances such as carbon monoxide or sulfur compounds can:
Block active sites on the catalyst
Reduce the rate of electrode reactions
This lowers the voltage and efficiency of the fuel cell, making high-purity hydrogen essential.
As a storage cell discharges, reactant concentrations change.
This leads to:
A decrease in the electrode potentials of the half-cells
Increased internal resistance due to product build-up
Both effects reduce the potential difference between the electrodes, causing the cell voltage to fall during use.
The main limitations are practical rather than chemical.
These include:
Difficulty and cost of producing hydrogen sustainably
Challenges in storing and transporting hydrogen safely
High cost of catalysts and fuel cell materials
These factors currently restrict large-scale adoption despite the favourable electrochemical principles.
Practice Questions
Explain why a hydrogen–oxygen fuel cell can produce a constant voltage for a long period of time, unlike a storage cell.
(2 marks)
Award up to 2 marks:
Fuel and oxygen are supplied continuously to the fuel cell. (1 mark)
The reactants are not used up inside the cell, so the cell reaction can continue without the voltage falling. (1 mark)
A hydrogen–oxygen fuel cell operates under standard conditions.
(a) State the oxidation reaction occurring at the anode.
(b) State the reduction reaction occurring at the cathode.
(c) Explain how electrode potentials are used to determine the voltage produced by the fuel cell.
(d) Give one advantage of a fuel cell compared with a storage cell, linked to its mode of operation.
(5 marks)
(a) Oxidation at the anode (1 mark):
Hydrogen is oxidised to form protons (or water in alkaline conditions) and electrons.
(b) Reduction at the cathode (1 mark):
Oxygen is reduced (gains electrons) to form water (or hydroxide ions in alkaline conditions).
(c) Use of electrode potentials (2 marks):
The voltage of the fuel cell is determined by the difference between the standard electrode potentials of the cathode and anode. (1 mark)
E°cell is calculated by subtracting the electrode potential of the anode from that of the cathode. (1 mark)
(d) Advantage of a fuel cell (1 mark):
Produces electricity continuously as long as fuel and oxygen are supplied / does not require recharging / produces low emissions when hydrogen is used.
Total: 5 marks

