OCR Specification focus:
‘Use standard electrode potentials, including hydrogen electrode, and measure cell potentials in various setups.’
Standard electrode potentials allow chemists to compare tendencies of half-cells to gain or lose electrons, enabling prediction of redox behaviour and construction of functional electrochemical cells.
Standard Electrode Potentials
Standard electrode potentials (E° values) provide a quantitative measure of how readily a species is reduced under standard conditions (1.0 mol dm⁻³ solutions, 298 K, 100 kPa). They allow half-reactions to be compared on a common scale so that feasible electron-transfer processes can be predicted. When a species is first described as a reducing or oxidising agent, its behaviour is assessed using these values.
Standard Electrode Potential (E°): The potential difference of a half-cell measured relative to the standard hydrogen electrode under standard conditions.
To understand E° values, students must be familiar with the standard hydrogen electrode (SHE), which is assigned a value of 0.00 V and acts as the universal reference point. It enables all other half-cells to be measured consistently by pairing them in a complete electrochemical cell.
The Standard Hydrogen Electrode (SHE)
The SHE consists of several essential features that maintain standard conditions and allow reproducible measurements.
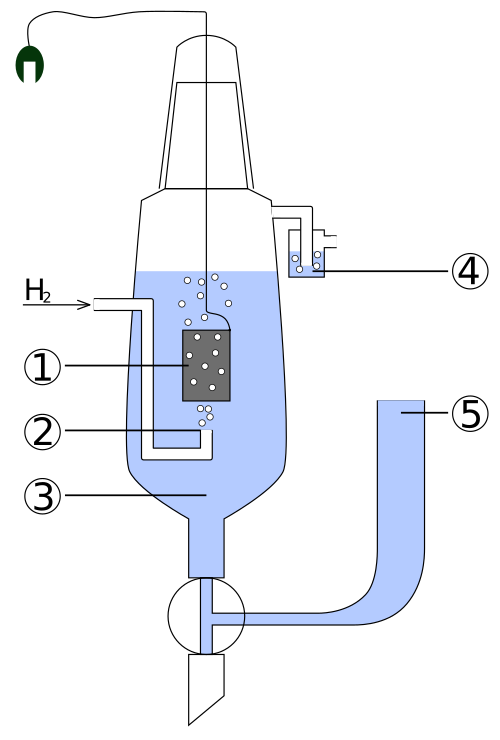
This diagram shows the standard hydrogen electrode (SHE), with hydrogen gas at 100 kPa in contact with 1.0 mol dm⁻³ H⁺(aq) on an inert platinised platinum electrode. The electrode is assigned an E° value of 0.00 V, providing a universal reference for all standard electrode potentials. Some construction details shown are practical features not required by the OCR specification. Source
It is used in every data table of standard electrode potentials.
Key features of the SHE include:
A platinum electrode, coated with finely divided platinum to increase surface area.
H₂ gas at 100 kPa bubbled over the electrode.
1.0 mol dm⁻³ H⁺(aq), usually provided by a strong acid.
Temperature maintained at 298 K.
Inert platinum, which conducts electrons but does not participate chemically.
This electrode behaves as both an oxidising and reducing half-cell depending on the species it is paired with, establishing a universal reference point on the electrochemical scale.
Measuring Standard Electrode Potentials
To measure an electrode potential, the half-cell of interest is connected to the SHE using a salt bridge, forming a complete circuit. The voltmeter reading corresponds to the standard electrode potential of the half-cell under investigation.
Salt bridges typically contain aqueous solutions of KNO₃ or KCl, selected to avoid introducing new ions that would participate in redox reactions.
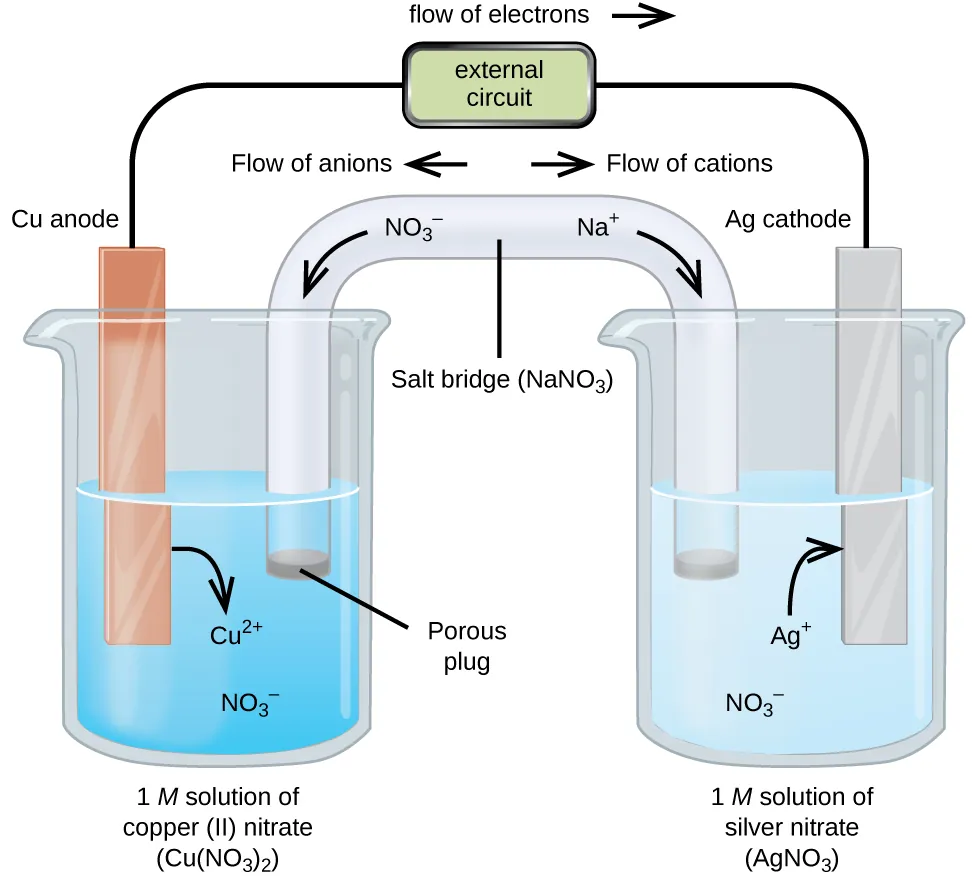
This diagram illustrates a galvanic cell with two half-cells connected by a salt bridge, showing electron flow from anode to cathode through the external circuit. The movement of ions through the salt bridge maintains electrical neutrality as the redox reaction proceeds. The salt bridge is labelled NaNO₃ here, which is equivalent in function to KNO₃ or KCl commonly referenced by OCR. Source
They allow ion flow to maintain electrical neutrality while preventing mixing of solutions.
Cell Potential (E°cell) = E°(positive electrode) − E°(negative electrode)
E°cell = Standard cell potential in volts (V)
E°(positive electrode) = Standard reduction potential of the electrode where reduction occurs
E°(negative electrode) = Standard reduction potential of the electrode where oxidation occurs
Electrons always flow from the more negative E° value to the more positive E° value, determining the direction of electron movement and allowing predictions of feasible redox reactions.
A brief sentence must separate this explanation from later definitions, ensuring clarity.
Constructing Electrochemical Cells
Electrochemical cells combine two half-cells to generate an electrical potential. This potential arises from the difference in electron-affinity between the two half-reactions.
Key components of a typical cell:
Two electrodes (metal or inert conductors)
Two electrolyte solutions, each containing ions of the electrode’s species
A salt bridge to complete the circuit
External wire and voltmeter to measure potential difference
The electrode with the more positive E° value acts as the cathode (where reduction occurs), and the electrode with the more negative value acts as the anode (where oxidation occurs).
Steps for cell construction:
Write the half-equations for each half-cell in their standard reduction form.
Identify the half-cell with the more positive E° value; this becomes the cathode.
Place the anode on the left and the cathode on the right in cell notation.
Connect the solutions with a salt bridge and measure the potential.
These steps reflect standard conventions used in data books and examinations.
Interpreting Standard Electrode Potentials
Electrode potentials help chemists predict the behaviour of oxidising and reducing agents.
General rules for interpretation:
A more positive E° value means the species is a stronger oxidising agent and more readily gains electrons.
A more negative E° value means the species is a stronger reducing agent and more readily loses electrons.
Electrons move from lower to higher E° values in an operating cell.
A reaction is feasible (thermodynamically) if the combined E°cell is positive.
These qualitative rules underpin much of redox chemistry, providing a framework for comparing electron-transfer tendencies between different half-cells.
Measuring Cell Potentials in Different Setups
The OCR specification requires awareness of how cell potentials can be measured in various configurations. While the standard setup uses the SHE, in practice any two half-cells can be paired to measure their overall potential.
Possible arrangements include:
Metal/metal ion half-cells (e.g., Zn/Zn²⁺, Cu/Cu²⁺)
Gas electrodes (e.g., H₂/H⁺ or Cl₂/Cl⁻) using platinum as an inert conductor
Ion/ion half-cells, where ions of the same element in different oxidation states are in solution with an inert electrode
These setups reflect the many types of redox systems encountered in applied and industrial chemistry.
Practical Considerations
To ensure accurate and reproducible electrode potential measurements, several practical factors must be controlled:
Concentration effects: Deviations from 1.0 mol dm⁻³ alter equilibrium positions and thus measured potentials.
Temperature: Potentials are temperature-dependent, so deviations from 298 K must be avoided.
Gas pressure: Gas-based half-cells must maintain 100 kPa partial pressure for standard conditions.
Electrode surface condition: Platinum electrodes must be clean and free of contaminants.
These factors ensure that students apply the concept of standard conditions accurately when using data from electrode potential tables.
FAQ
Platinum black is used to increase the surface area of the electrode.
A larger surface area allows hydrogen gas molecules to adsorb more effectively, improving contact between H₂, H⁺ ions and the electrode surface.
This ensures faster establishment of equilibrium and more stable, reproducible electrode potential readings.
Using reduction potentials provides a consistent reference system for all half-cells.
This avoids ambiguity when comparing different half-reactions and ensures that E° values can be directly compared and combined.
Oxidation potentials can be inferred by reversing the half-equation and changing the sign of the E° value.
The salt bridge must not participate in redox reactions.
Inert electrolytes such as potassium nitrate or potassium chloride:
Do not react with ions in the half-cells
Provide mobile ions to maintain charge balance
Prevent mixing of the two solutions
This ensures the measured cell potential reflects only the intended redox processes.
Some redox systems involve only ions or gases and no solid conducting material.
Inert electrodes, such as platinum, are used to:
Conduct electrons without reacting
Provide a surface for electron transfer
Allow half-cells like Fe³⁺/Fe²⁺ or Cl₂/Cl⁻ to function
The electrode itself does not affect the electrode potential.
Electrode potentials depend on temperature, concentration and gas pressure.
Fixing conditions at:
298 K
1.0 mol dm⁻³ concentrations
100 kPa gas pressure
Allows reliable comparison of E° values between different half-cells and ensures data consistency across experiments and data tables.
Practice Questions
Define the standard electrode potential of a half-cell and state the standard conditions under which it is measured.
(2 marks)
Definition includes reference to measurement of a half-cell potential relative to the standard hydrogen electrode (1 mark)
Correct statement of standard conditions, including any two of:
1.0 mol dm⁻³ solutions
298 K
100 kPa pressure
(1 mark)
A student constructs an electrochemical cell using a Zn²⁺/Zn half-cell and a Cu²⁺/Cu half-cell under standard conditions.
(a) Identify which electrode acts as the anode and which acts as the cathode.
(b) Describe the direction of electron flow in the external circuit.
(c) Explain the role of the salt bridge in this cell.
(5 marks)
(a)
Zinc electrode identified as the anode (oxidation occurs) (1 mark)
Copper electrode identified as the cathode (reduction occurs) (1 mark)
(b)
Electrons flow from the zinc electrode to the copper electrode through the external circuit (1 mark)
(c)
Salt bridge allows movement of ions to maintain electrical neutrality in each half-cell (1 mark)
Prevents charge build-up and allows the cell to continue operating / completes the circuit (1 mark)
Maximum 5 marks.

