OCR Specification focus:
‘Identify electromagnetic spectrum regions and shared wave properties.’
The electromagnetic (EM) spectrum encompasses all types of electromagnetic radiation, from radio waves to gamma rays, united by their shared wave properties. Understanding their characteristics and relative positions on the spectrum is fundamental to appreciating how energy, frequency, and wavelength interrelate across a vast range of phenomena in physics and everyday applications.
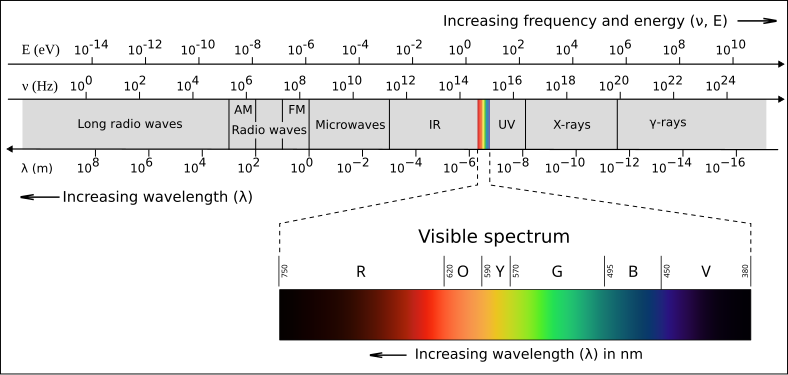
A labelled electromagnetic spectrum showing the standard regions from radio to gamma rays, with corresponding wavelength, frequency, and photon energy scales. This visual reinforces that all EM radiation forms a continuous spectrum and highlights the inverse relationship between wavelength and frequency. Some versions also indicate approximate visible range boundaries. Source.
The Nature of Electromagnetic Waves
All electromagnetic waves consist of oscillating electric and magnetic fields that travel through space. These oscillations are mutually perpendicular and also perpendicular to the direction of wave propagation, making EM waves transverse in nature.
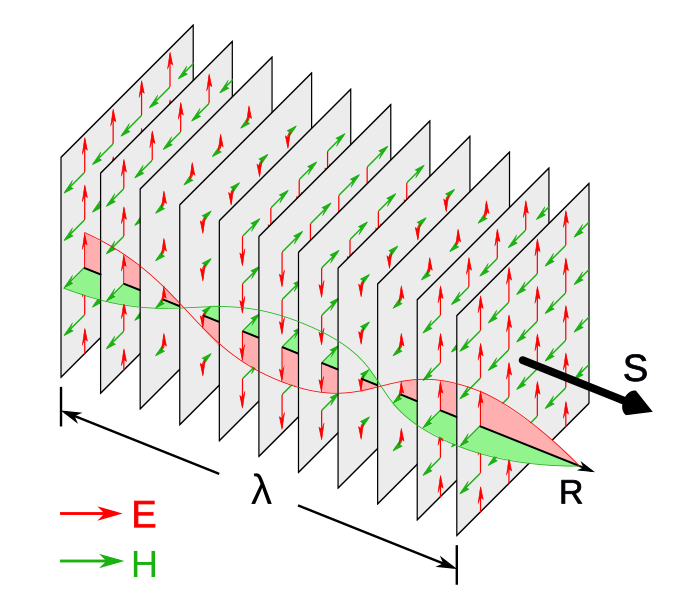
Diagram of a linearly polarised plane electromagnetic wave showing the electric field and magnetic field oscillations at right angles to each other and to the direction of travel. This clarifies why all EM waves are classified as transverse. The simple layout avoids extra details, focusing solely on geometry and orientation. Source.
Electromagnetic Wave: A transverse wave consisting of oscillating electric and magnetic fields that propagate through space, carrying energy without the need for a medium.
Unlike sound or water waves, EM waves do not require a physical medium; they can travel through a vacuum. This property is critical for understanding how sunlight reaches Earth and how radio communications operate in space.
EQUATION
—-----------------------------------------------------------------
Wave Speed (c) = Frequency (f) × Wavelength (λ)
c = 3.00 × 10⁸ m s⁻¹ in a vacuum
f = Number of oscillations per second (Hz)
λ = Distance between corresponding points on successive wave cycles (m)
—-----------------------------------------------------------------
Every form of electromagnetic radiation follows this fundamental relationship. Because the speed of light (c) is constant in a vacuum, increasing the frequency results in a proportional decrease in wavelength.
Shared Properties of Electromagnetic Waves
All EM waves share the following key characteristics:
Travel at the same speed in a vacuum: approximately 3.00 × 10⁸ m s⁻¹.
Can be reflected, refracted, diffracted, and polarised.
Transfer energy from one location to another without transferring matter.
Obey the wave equation c=fλc = fλc=fλ.
Exhibit interference and diffraction, typical of all wave phenomena.
Do not require a medium, allowing transmission through empty space.
Can be absorbed and emitted by atoms, leading to various physical effects like photoelectric emission and atomic excitation.
Each of these features reflects the dual wave-particle nature of light, though this subsubtopic focuses purely on wave behaviour.
The Electromagnetic Spectrum
The electromagnetic spectrum represents the complete range of electromagnetic radiation types, arranged in order of increasing frequency (and decreasing wavelength). Although the boundaries between regions are not fixed, physicists categorise them by frequency range and general behaviour of interaction with matter.
From lowest to highest frequency, the spectrum includes:
Radio Waves
Longest wavelengths, ranging from kilometres to millimetres.
Generated by oscillating currents in electrical circuits.
Used for broadcasting, communication, and radar.
Microwaves
Wavelengths between about 1 m and 1 mm.
Produced by electronic oscillators and molecular transitions.
Applications in satellite transmission, radar, and microwave ovens.
Infrared (IR) Radiation
Wavelengths just longer than visible light, from 1 mm to 700 nm.
Emitted by warm objects; associated with heat energy.
Used in remote controls, thermal imaging, and spectroscopy.
Visible Light
Wavelengths approximately from 700 nm (red) to 400 nm (violet).
The only portion detectable by the human eye.
Enables vision, photography, and optical fibre communication.
Ultraviolet (UV) Radiation
Wavelengths from about 400 nm down to 10 nm.
Produced by high-energy electron transitions in atoms.
Can cause ionisation, sunburn, and fluorescence.
X-rays
Wavelengths between roughly 10 nm and 0.01 nm.
Generated when high-speed electrons are decelerated by metal targets.
Highly penetrating; used in medical imaging and security scanning.
Gamma Rays
Shortest wavelengths, less than 0.01 nm.
Originating from nuclear decay and cosmic phenomena.
Extremely energetic; used in radiotherapy and sterilisation.
Between these regions, properties change continuously. There is no sharp boundary between one region and the next; instead, transitions occur gradually as frequency and energy increase.
Relationships Between Energy, Frequency, and Wavelength
As frequency increases across the spectrum, so does photon energy, while wavelength decreases. This relationship is fundamental in understanding the effects and applications of different EM wave types.
EQUATION
—-----------------------------------------------------------------
Photon Energy (E) = Planck’s Constant (h) × Frequency (f)
h = 6.63 × 10⁻³⁴ J s
f = Frequency of radiation (Hz)
E = Energy of one photon (J)
—-----------------------------------------------------------------
This shows that higher-frequency radiation, such as X-rays and gamma rays, carries greater energy per photon than lower-frequency radiation such as radio or infrared waves. Consequently, their ability to ionise atoms and damage biological tissue increases.
The Spectrum in Context
The electromagnetic spectrum is continuous and vast, spanning over 20 orders of magnitude in frequency. Despite their differences, all EM waves share identical physical foundations — they are all disturbances of electric and magnetic fields propagating at light speed.
Practical applications exploit specific regions based on their frequency and energy characteristics:
Communication technologies use radio and microwaves because they penetrate the atmosphere and carry information efficiently.
Infrared and visible light are essential for energy transfer and optical imaging.
Ultraviolet, X-rays, and gamma rays serve roles in sterilisation, medicine, and research due to their high energy.
Wave–Particle Duality and Energy Transmission
Though discussed more deeply elsewhere, it is worth noting that electromagnetic radiation exhibits wave–particle duality. This means that while it behaves as a wave, transmitting energy continuously through space, it can also act as discrete quanta of energy called photons. Each photon carries an energy amount determined by its frequency, consistent with Planck’s equation above.
This duality underpins quantum mechanics and reinforces why the electromagnetic spectrum is central to understanding both classical and modern physics — connecting the macroscopic behaviour of waves with microscopic quantum phenomena.
FAQ
Absorption and transmission depend on how the energy of the electromagnetic waves interacts with the electronic and molecular structure of the material.
Low-energy radiation (radio, microwaves) often passes through most materials because it doesn’t match electronic energy levels.
Infrared is absorbed by molecular vibrations in many materials, producing heat.
Visible and ultraviolet interact with electronic transitions, leading to colour and fluorescence.
X-rays and gamma rays are so energetic they can penetrate most materials, but dense substances such as lead absorb them effectively.
Each type of electromagnetic wave has a frequency that determines the design and scale of the device used to generate or detect it.
Radio antennas are typically comparable in size to the wavelength (metres long).
Microwave dishes are smaller and more precisely shaped to focus shorter wavelengths.
Optical detectors, such as photodiodes, rely on quantum interactions where individual photons produce electronic signals.
The principle is that the device must resonate or respond efficiently at the wave’s frequency.
Whenever an electric charge accelerates—changing its speed or direction—it disturbs the surrounding electric and magnetic fields. This disturbance propagates outward as an electromagnetic wave.
For example:
Alternating currents in antennas create oscillating electric and magnetic fields that radiate as radio waves.
Electrons in atoms transitioning between energy levels emit electromagnetic radiation at specific frequencies, such as visible light or X-rays.
The frequency of the emitted wave matches the frequency of the charge oscillation or the energy difference in atomic transitions.
The divisions of the spectrum (radio, microwave, infrared, etc.) are purely for convenience. There are no physical gaps between one region and the next.
Wavelength and frequency change smoothly, and the same fundamental laws apply across the whole range.
What differs is how each frequency range interacts with matter — for example, visible light excites electrons, whereas microwaves cause molecular rotation.
Thus, while regions have different names and uses, they are part of one continuous distribution of electromagnetic radiation.
The higher the photon energy, the more capable it is of breaking molecular bonds or ionising atoms in living tissue.
Low-energy photons (radio, infrared) primarily cause heating by vibration or rotation of molecules.
Mid-energy photons (visible and UV) can trigger chemical changes; UV can damage DNA.
High-energy photons (X-rays, gamma rays) can ionise atoms and lead to radiation damage.
This relationship underpins safety standards, determining exposure limits for various electromagnetic sources.
Practice Questions
Question 1 (2 marks)
State two properties that are shared by all electromagnetic waves.
Mark scheme:
1 mark for each correct property (any two of the following):
• They are transverse waves.
• They all travel at the same speed in a vacuum (3.0 × 10⁸ m s⁻¹).
• They can be reflected, refracted, diffracted, and polarised.
• They transfer energy without transferring matter.
• They do not require a medium to travel through.
Question 2 (5 marks)
Describe and explain how the properties of electromagnetic radiation change across the electromagnetic spectrum from radio waves to gamma rays. Include reference to wavelength, frequency, and energy, and link your explanation to their practical applications.
Mark scheme:
1 mark for stating that as you move from radio waves to gamma rays, wavelength decreases.
1 mark for stating that frequency increases across the same direction.
1 mark for stating that photon energy increases (E = hf).
1 mark for explaining that increasing frequency (and therefore energy) means higher-energy waves such as X-rays and gamma rays can ionise atoms or penetrate materials more deeply.
1 mark for linking these trends to applications — e.g. radio waves for communication (low energy, long wavelength) and gamma rays for sterilisation or medical imaging (high energy, short wavelength).

