AP Syllabus focus:
‘Explain amino acid structure, including central carbon, functional groups, and variable R group categorized as nonpolar, polar, or ionic, whose interactions affect protein structure and function.’
Amino acids are the building blocks of proteins. Their shared core structure creates predictable chemical behavior, while differences in the R group create diverse properties that shape how proteins fold, bind, and function.
Core Amino Acid Structure
All amino acids share the same basic “backbone” with one variable component that determines identity.
Amino acid: An organic monomer with a central (alpha) carbon bonded to an amino group, a carboxyl group, a hydrogen atom, and a variable side chain (R group).
The Alpha (Central) Carbon and Four Attachments
The central carbon (alpha carbon) is bonded to four different groups in most amino acids:
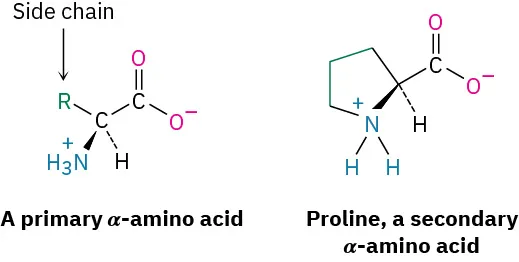
This diagram highlights the generic α-amino acid backbone with the variable side chain labeled “R,” making it easy to identify the common structural template shared by all amino acids. It also contrasts that template with proline, where the nitrogen is part of a ring, illustrating an important structural exception that influences protein structure. Source
Amino group (commonly written as –NH2, but often protonated in cells)
Carboxyl group (commonly written as –COOH, but often deprotonated in cells)
Hydrogen atom
R group (side chain), which differs among amino acids
Because the alpha carbon often has four different attachments, most amino acids are chiral, meaning they can exist as two mirror-image forms; biological proteins are built almost exclusively from the L form.
Functional Groups and Ionisation (Charge)
The amino and carboxyl functional groups can gain or lose H+ depending on pH.
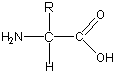
This instructional page includes a generic amino acid structure diagram and explains how the amino group tends to become protonated while the carboxyl group tends to become deprotonated near physiological pH. It connects the structural features to the zwitterion concept, which is essential for predicting solubility and interaction behavior. Source
In typical cellular conditions, amino acids often exist as zwitterions (having both a positive and a negative charge), which:
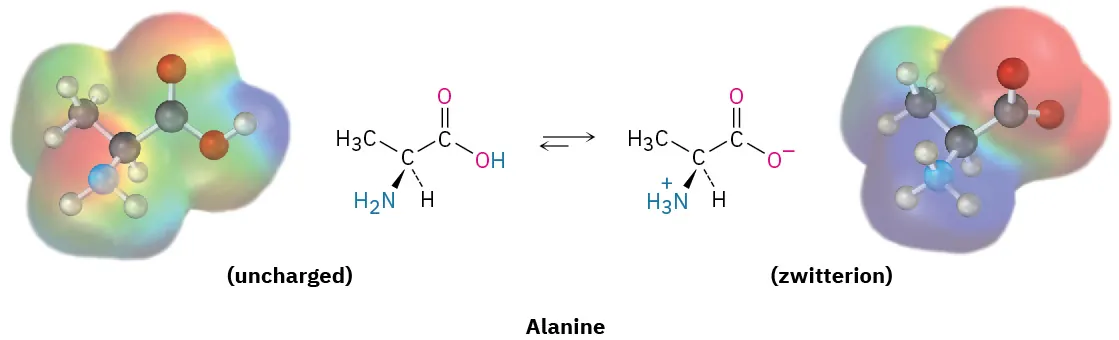
Alanine is shown shifting between an uncharged form and its zwitterionic form, highlighting proton transfer that produces and at typical cellular pH. The paired electrostatic potential surfaces visually connect charge distribution to polarity and water interactions. Source
Affects solubility in water
Influences how amino acids (and proteins) interact with charged molecules
Helps explain why many amino acids behave as buffers within certain pH ranges
The R Group (Side Chain) and Its Categories
The R group is the primary source of chemical diversity among amino acids and is the key determinant of how each amino acid behaves in a protein.
R group (side chain): The variable chemical group attached to an amino acid’s alpha carbon that determines the amino acid’s polarity, charge, and typical interactions.
R groups are commonly categorized as nonpolar, polar, or ionic (charged). These categories help predict how an amino acid will interact with water and with other parts of a protein.
Nonpolar (Hydrophobic) R Groups
Nonpolar amino acids have R groups dominated by hydrocarbons, making them hydrophobic.
Tend to cluster away from water in aqueous environments
Commonly found in the interior of globular proteins
Promote folding patterns driven by the hydrophobic effect (water “pushes” nonpolar groups together)
Polar (Uncharged) R Groups
Polar amino acids have R groups with electronegative atoms (often O or N) that create partial charges.
Typically hydrophilic and more compatible with water
Often found on protein surfaces exposed to the cytosol or extracellular fluid
Can form hydrogen bonds, stabilising specific shapes and enabling specific binding to other molecules
Ionic (Charged) R Groups: Acidic and Basic
Some R groups carry a full charge at physiological pH.
Acidic side chains tend to be negatively charged after donating H+
Basic side chains tend to be positively charged after accepting H+ Charged R groups:
Increase water solubility
Can form ionic attractions with oppositely charged groups
Strongly affect protein behaviour when environmental pH changes (because charge states can shift)
How R Group Interactions Affect Protein Structure and Function
Protein properties emerge from the combined behavior of many amino acids, especially how their R groups interact.
Interaction Types Driven by R Groups
Hydrophobic interactions: nonpolar R groups pack together, influencing overall folding in water
Hydrogen bonding: polar R groups (and sometimes backbone atoms) form specific attractions that help maintain shape and enable recognition
Ionic interactions: oppositely charged R groups attract, affecting stability and responsiveness to pH and salt concentration
Functional Consequences
R group chemistry helps determine:
Protein shape (which influences what a protein can do)
Binding specificity (which molecules a protein can interact with)
Activity in different environments (changes in pH or ion concentration can alter charges and interactions)
Local chemical microenvironments at active or binding sites, where certain R groups can participate in acid-base behaviour or stabilise charges
FAQ
Chirality arises because the alpha carbon is usually bonded to four different groups.
Proteins are synthesised by enzymes that are stereospecific, so they incorporate L-amino acids almost exclusively.
At low pH, groups are more likely to gain H+ (more protonated).
At high pH, groups are more likely to lose H+ (more deprotonated).
This shifts net charge and can alter interactions driven by R groups.
Polar R groups are generally hydrophilic, but the extent varies with size and exact chemistry.
Some amino acids have mixed features, so behaviour depends on the surrounding protein environment.
Acidic R groups tend to donate H+ and become negatively charged.
Basic R groups tend to accept H+ and become positively charged.
Whether they are charged depends on local pH and the microenvironment in the folded protein.
The local environment can shift how exposed an R group is to water and can alter its effective charge.
Nearby residues, ions, and local polarity can strengthen or weaken hydrogen bonding and ionic attractions.
Practice Questions
State the four groups attached to the central carbon of an amino acid. (2 marks)
Amino group (1)
Carboxyl group (1)
Hydrogen and R group may be credited in place of either, up to max 2
Explain how differences in amino acid R groups influence protein structure and function. In your answer, refer to nonpolar, polar, and ionic R groups and the interactions they enable. (6 marks)
Nonpolar R groups are hydrophobic and tend to cluster away from water (1)
This promotes folding/packing that affects overall protein shape (1)
Polar (uncharged) R groups are hydrophilic and can form hydrogen bonds (1)
Hydrogen bonding contributes to stabilising specific conformations and/or binding specificity (1)
Ionic R groups carry positive or negative charge (1)
Ionic attractions and changes in charge with pH/ions affect stability and function (1)

