AP Syllabus focus:
‘Explain how hydrogen bonding within the polypeptide backbone produces secondary structures such as alpha-helices and beta-pleated sheets.’
Proteins begin as linear amino acid chains, but local, repeating folding patterns quickly form. These patterns, called secondary structure, arise from predictable hydrogen bonding within the backbone and strongly influence how a protein can fold further.
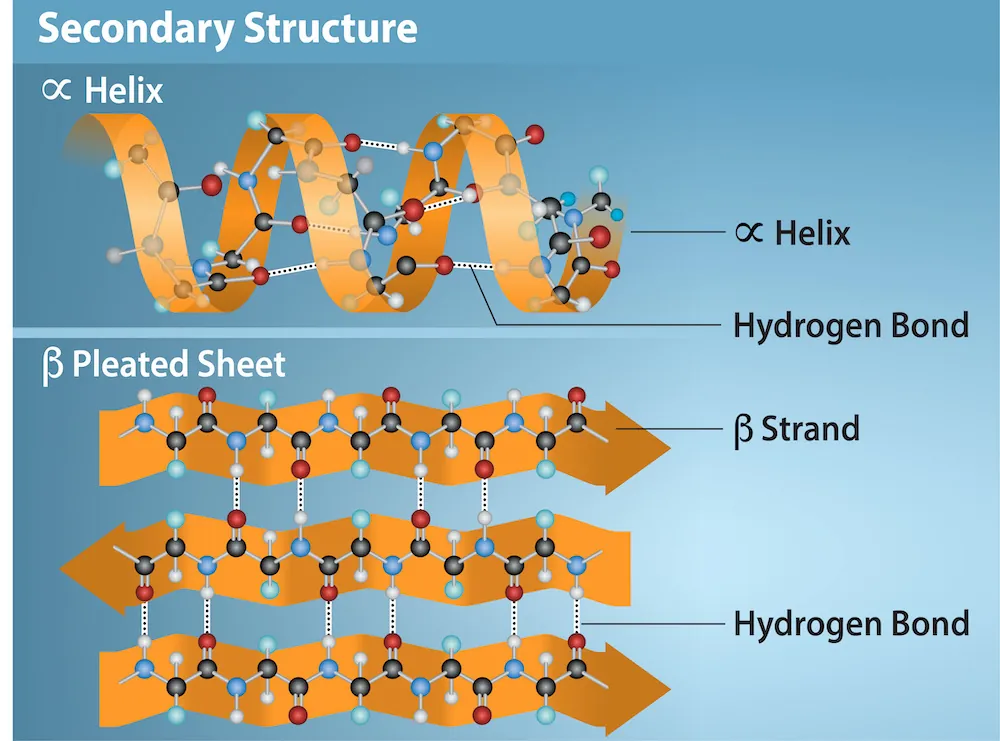
This figure summarizes the two major secondary structures—an α-helix and a β-pleated sheet—and highlights that both are stabilized by hydrogen bonds formed between backbone carbonyl oxygens (C=O) and backbone amide hydrogens (O=C-N-HR). It reinforces that the repeating backbone (not the R groups) provides the donors and acceptors that create regular, predictable folding patterns. Source
What “Secondary Structure” Means
Secondary structure refers to regular, repeating folding patterns in a polypeptide that are stabilized by hydrogen bonds between atoms in the polypeptide backbone (not the R groups).
Secondary structure: Local, repeating folding of a polypeptide backbone (primarily alpha-helices and beta-pleated sheets) stabilised by hydrogen bonding between backbone C=O and N–H groups.
This idea is central to the syllabus focus: hydrogen bonding within the polypeptide backbone is what generates these structures.
Why the Backbone can Hydrogen-Bond
Every peptide bond creates a repeating backbone pattern with:
A carbonyl group (C=O) that can act as a hydrogen bond acceptor (oxygen)
An amide group (N–H) that can act as a hydrogen bond donor (hydrogen)
Because these groups repeat along the chain, a polypeptide has many opportunities to form backbone-to-backbone hydrogen bonds, generating stable, repeating shapes.
Alpha-Helices
An alpha-helix is a coiled, spiral secondary structure resembling a spring.
How Hydrogen Bonds Stabilise an Alpha-Helix
The helix is stabilised by intrachain hydrogen bonds (within the same polypeptide strand).
A typical pattern is backbone C=O of one amino acid hydrogen-bonding to backbone N–H of an amino acid a few residues ahead in the chain.
These repeated hydrogen bonds align in the same general direction, creating a strong stabilising effect along the helix.
Key Features to Know
The R groups project outward from the helix, reducing crowding in the core and allowing side chains to interact with the surrounding environment.
Alpha-helices form readily because the backbone groups are positioned to maximise hydrogen bonding while maintaining a compact shape.
Helices can be amphipathic (one side more polar, the other more nonpolar) depending on the sequence; this helps explain why some helices sit at interfaces (for example, between watery and less watery regions).
Beta-Pleated Sheets
A beta-pleated sheet is a secondary structure made of extended polypeptide segments lying side-by-side, forming a “pleated” surface.
How Hydrogen Bonds Stabilise Beta-Sheets
Beta-sheets are stabilised by hydrogen bonds between adjacent strands of polypeptide backbone.
Hydrogen bonds form between a backbone C=O on one strand and a backbone N–H on a neighbouring strand.
These bonds can link:
Different regions of the same polypeptide folded back on itself, or
Different polypeptide chains aligned next to one another
Parallel vs Antiparallel Arrangement (Conceptual)
The relative direction of the strands affects hydrogen bonding geometry:
Parallel beta-sheets: strands run in the same direction; hydrogen bonds are slightly offset.
Antiparallel beta-sheets: strands run in opposite directions; hydrogen bonds tend to align more directly.
AP Biology typically emphasises recognising that both are forms of beta-sheets and that hydrogen bonding within the backbone is the stabilising force.
Key Features to Know
R groups alternate above and below the sheet, which can create distinct “sides” with different chemical properties.
The “pleated” appearance results from the angles in the backbone bonds, not from side-chain interactions.
What Secondary Structure Depends on (and What it Does Not)
Secondary structure formation is largely driven by the backbone’s ability to hydrogen-bond, but the amino acid sequence still matters because it affects how easily the backbone can adopt these shapes.
Depends primarily on:
Availability and alignment of backbone C=O and N–H groups for hydrogen bonding
Local backbone flexibility and steric constraints
Not primarily caused by:
Direct hydrogen bonding between R groups (that is more associated with other structural effects)
Disruption of Secondary Structure
Because alpha-helices and beta-sheets rely on hydrogen bonds, they can be disrupted when conditions interfere with those interactions, such as:
Temperature increases that raise molecular motion
Changes in pH that alter the ability of groups to participate in hydrogen bonding
FAQ
Different side-chain sizes and branching can make a region more or less compatible with tight coiling or extended strands. Bulky or awkwardly shaped R groups can hinder one pattern and favour the other.
Yes, local regions can rearrange if hydrogen bonds are broken and re-formed in a new pattern. This is more likely if conditions change or if the sequence can support both conformations.
It’s a helix with polar/charged R groups mostly on one side and nonpolar R groups on the other.
Often forms when residues repeat in a pattern matching the helix’s rotation.
The backbone bond angles and geometry force a slight zig-zag in the strands. When many residues align, this creates a rippled, pleated surface across the sheet.
Common approaches include:
X-ray crystallography or cryo-EM to visualise backbone arrangement
Circular dichroism spectroscopy to estimate helix vs sheet content from characteristic signals
Practice Questions
State what type of bonding stabilises alpha-helices and beta-pleated sheets in proteins. (2 marks)
Hydrogen bonding (1)
Between groups in the polypeptide backbone (C=O and N–H), not between R groups (1)
Describe how hydrogen bonding within a polypeptide backbone produces (i) an alpha-helix and (ii) a beta-pleated sheet. (5 marks)
Alpha-helix is a coiled/spiral structure of a single polypeptide region (1)
Stabilised by hydrogen bonds within the same chain (intrachain) (1)
Hydrogen bonds form between backbone C=O and backbone N–H groups (1)
Beta-pleated sheet consists of extended strands aligned side-by-side (1)
Stabilised by hydrogen bonds between neighbouring strands’ backbones (interstrand), again between C=O and N–H groups (1)

