AP Syllabus focus:
‘Describe how hydrogen bonds, hydrophobic interactions, ionic interactions, and disulfide bridges stabilize the three-dimensional tertiary structure of proteins.’
Tertiary structure is where a polypeptide becomes a functional 3D molecule.
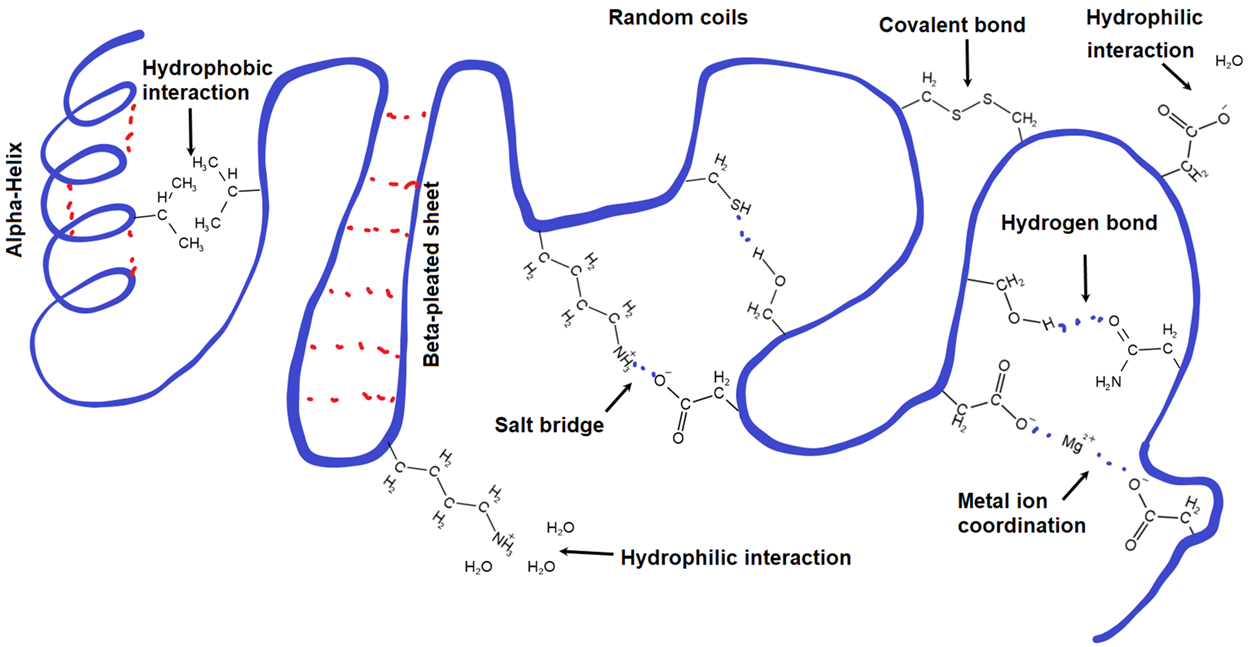
This diagram summarizes the major interaction types that stabilize protein tertiary structure, including hydrogen bonding, hydrophobic interactions, ionic “salt bridges,” and disulfide linkages. Seeing all of these forces on a single folded polypeptide helps connect the idea of a 3D fold to the specific chemical attractions between side chains. Source
It is stabilised by multiple chemical interactions among side chains and the backbone, all influenced by the aqueous cellular environment.
What Tertiary Structure Describes
Tertiary structure: The overall three-dimensional shape of a single polypeptide produced by interactions among amino acid side chains (R groups) and, to a lesser extent, the polypeptide backbone.
Tertiary structure reflects how different R groups interact once the chain has folded, creating domains and pockets that determine biological activity.
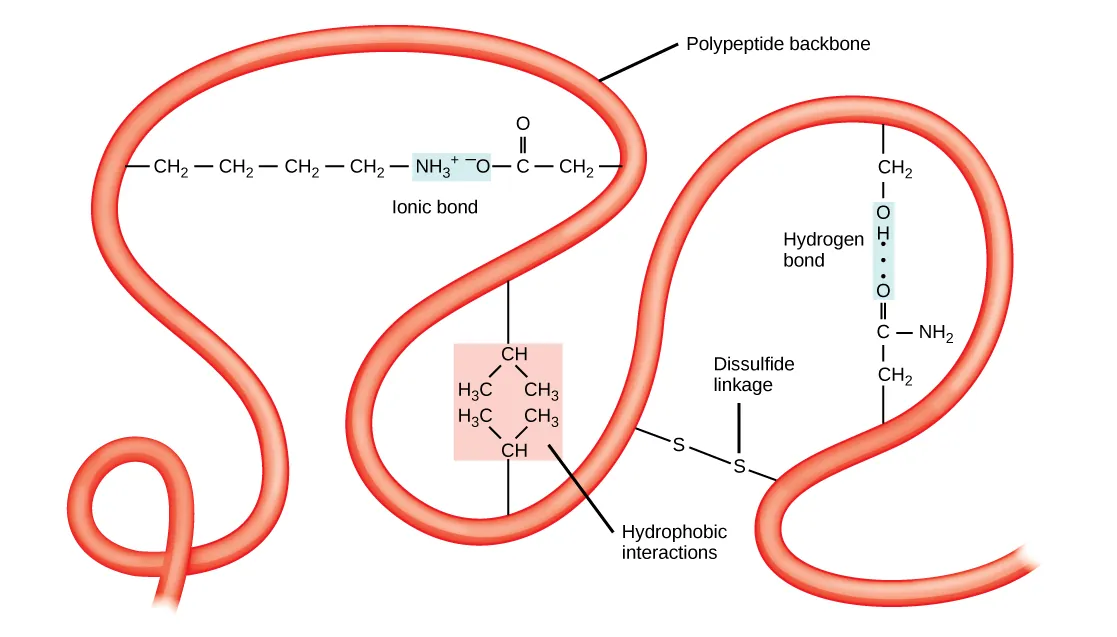
This figure depicts a folded polypeptide and labels representative stabilizing interactions: hydrophobic clustering of nonpolar side chains, ionic bonding between charged side chains, hydrogen bonding between polar groups, and a covalent disulfide linkage. It reinforces that tertiary structure is a consequence of side-chain chemistry (R groups) interacting within an aqueous environment. Source
Stabilising interactions in tertiary structure
Hydrogen Bonds
Hydrogen bonds form when a hydrogen covalently bonded to an electronegative atom (often O or N) is attracted to another electronegative atom.
Commonly occur between polar R groups (for example, -OH, -NH) and other polar groups or water.
Provide specificity: many weak bonds together strongly stabilise a particular fold.
Can be disrupted by changes in pH or temperature that alter charges or molecular motion.
Hydrophobic Interactions
Hydrophobic interactions are driven by water: nonpolar groups cluster to minimise contact with water, increasing overall stability.
Nonpolar R groups (often rich in C–H) tend to pack into a hydrophobic core.
This “core” helps set the protein’s internal architecture and positions polar/charged groups toward the surface.
Although not a single bond type, the effect is a major stabiliser because it reduces energetically unfavourable ordering of surrounding water molecules.
Ionic Interactions (Salt Bridges)
Ionic interactions occur between oppositely charged side chains, often forming salt bridges.
Typically involve acidic R groups (negatively charged) and basic R groups (positively charged).
Stronger than many individual hydrogen bonds, but highly dependent on environment:
Shifts in pH can change protonation state, removing charges and breaking ionic attractions.
High ionic strength solutions can “shield” charges, weakening attractions.
Disulfide Bridges
A disulfide bridge is a covalent bond formed between two cysteine side chains, linking distant parts of a polypeptide.
Disulfide bridge: A covalent S–S bond formed when two cysteine R groups are oxidised, stabilising a protein’s folded shape by cross-linking regions of the polypeptide.
Disulfide bridges are especially important for proteins that function outside the cell, where conditions can be more variable.
They “lock in” folds, increasing resistance to unfolding.
Reducing conditions can break disulfide bridges, destabilising the tertiary structure.
How Conditions Influence Tertiary Stability (Without Changing Sequence)
Tertiary structure can be altered without changing the amino acid sequence because the stabilising forces depend on the surrounding chemistry.
Temperature increases can disrupt hydrogen bonds and weaken hydrophobic packing by increasing molecular motion.
pH changes can disrupt ionic interactions by changing side-chain charges, and can also alter hydrogen bonding patterns.
Oxidising vs reducing environments determine whether disulfide bridges form or break.
When these stabilising interactions are sufficiently disrupted, proteins may denature (unfold or misfold), often losing function because the precise 3D arrangement of key R groups is required for binding and catalysis.
Functional Consequences of Tertiary Structure
The 3D fold positions specific side chains to create functional sites.
Active sites in enzymes require correct alignment of catalytic R groups and stabilising interactions with substrates.
Binding specificity (for receptors, antibodies, transport proteins) depends on complementary shape and chemical properties produced by tertiary folding.
Small disruptions to stabilising interactions can change the shape/charge of a binding pocket, reducing activity even if the primary sequence remains unchanged.
FAQ
No. They are an emergent effect of water excluding nonpolar groups, indirectly stabilising folding through nonpolar clustering.
In more oxidising environments (often secretory pathways/extracellular spaces), which favour cysteine oxidation to form S–S links.
Yes. Salt bridges often form on or near surfaces and can also stabilise local folding within buried regions if charges are properly paired.
Their combined effect is large: breaking the fold would require disrupting numerous hydrogen bonds, ionic attractions, and hydrophobic packing simultaneously.
A single amino acid change can remove a charge, add polarity, or remove a cysteine, disrupting salt bridges, hydrogen bonding networks, hydrophobic packing, or disulphide cross-links.
Practice Questions
Explain how hydrophobic interactions contribute to the tertiary structure of a protein. (2 marks)
Nonpolar R groups cluster away from water / form a hydrophobic core. (1)
This minimises contact with water (or reduces unfavourable interactions) and stabilises the folded shape. (1)
A protein is moved to a solution of much lower pH and loses its normal shape. Describe how changes in hydrogen bonds, ionic interactions, and disulphide bridges could contribute to this loss of tertiary structure. (5 marks)
pH change alters protonation of side chains, changing charges on acidic/basic R groups. (1)
Loss of opposite charges disrupts ionic interactions/salt bridges. (1)
Changed protonation alters hydrogen bonding patterns or reduces hydrogen bond formation. (1)
Disruption of many weak interactions leads to unfolding/denaturation and loss of the normal 3D shape. (1)
Disulphide bridges are covalent cross-links that stabilise folding; if reducing conditions occur (or if bonds are otherwise disrupted), stability decreases. (1)

