AP Syllabus focus:
‘Describe how covalent peptide bonds form between amino acids’ carboxyl and amine groups to create linear polypeptide chains.’
Amino acids are the monomers of proteins. Their key chemistry involves forming covalent peptide bonds that link amino acids into linear polypeptide chains, establishing the backbone on which later protein structure is built.
Core Idea: Linking Amino Acids into a Chain
Proteins begin as polypeptides, which are unbranched chains of amino acids connected in a specific order. This order is created by repeated formation of the same covalent linkage between two functional groups on adjacent amino acids.
Amino Acids as Monomers
An amino acid has two essential reactive regions: an amino group and a carboxyl group. These groups participate directly in chain formation.
Amino acid: An organic monomer with both an amino group (–NH₂/–NH₃⁺) and a carboxyl group (–COOH/–COO⁻) that can be covalently linked to other amino acids to form polypeptides.
In cells, amino acids are typically in an aqueous environment, so the amino and carboxyl groups often occur in ionized forms (–NH₃⁺ and –COO⁻). Even when ionized, the same atoms participate in peptide bond formation.
The Functional Groups that React
The carboxyl group of one amino acid provides the carbonyl carbon (C=O) involved in the bond.
The amino group of a second amino acid provides the nitrogen (N) that becomes bonded to that carbonyl carbon.
The remainder of each amino acid (sometimes called the “side chain”) does not form the peptide bond itself, but remains attached to the backbone.
Peptide Bond Formation (Condensation)
Peptide bonds form by a specific covalent reaction between two amino acids: the carboxyl end of one reacts with the amine end of another.
Peptide bond: A covalent amide linkage (–C(=O)–NH–) formed when the carboxyl group of one amino acid reacts with the amino group of another, joining them into a growing chain.
This bond forms through a condensation (dehydration) reaction, meaning water is produced as the bond forms.
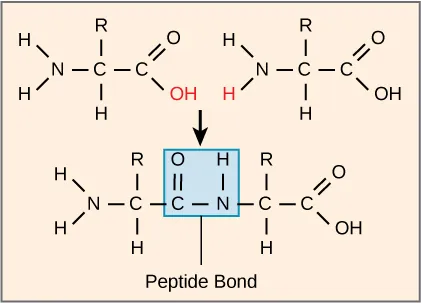
Peptide bond formation is shown as a dehydration (condensation) reaction: an –OH from the carboxyl group and an –H from the amino group combine to form water, while the carbonyl carbon bonds to the amino nitrogen. The highlighted linkage is the amide (peptide) bond, –C(=O)–NH–, that joins amino acids into a growing chain. Source
What is Removed and What is Joined
From the carboxyl group: an –OH is removed.
From the amino group: an –H is removed.
The removed –OH and –H combine to form H₂O.
The remaining carbonyl carbon bonds to the amino nitrogen, creating the –C(=O)–NH– peptide linkage.
What the Chain Looks Like
As peptide bonds form repeatedly, amino acids become arranged in a linear sequence (not a branched network). The repeating pattern of atoms along the chain is called the polypeptide backbone, consisting of alternating units that include the amide nitrogen and the carbonyl carbon.
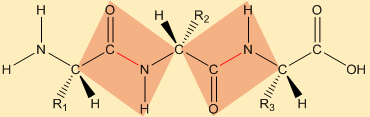
A short polypeptide segment is drawn to show the repeating backbone architecture created by peptide bonds, with side chains (R groups) branching off the backbone rather than forming the linkage. The shaded planes illustrate that each peptide bond has restricted rotation and a largely planar geometry, which is a key structural consequence of forming the –C(=O)–NH– bond. Source
Polypeptide: A linear polymer of amino acids covalently linked by peptide bonds, forming a single continuous chain.
A polypeptide has two different ends because different functional groups remain unreacted at the termini:
One end retains a free (unlinked) amino group: the N-terminus.
The other end retains a free (unlinked) carboxyl group: the C-terminus.
Why Peptide Bonds Matter Biologically
Peptide bond formation is fundamental because it:
Creates a stable covalent backbone that preserves the sequence of amino acids.
Produces a chain with consistent chemical features (repeating –C(=O)–NH– units), which influences how the chain can interact with itself and its environment.
Enables cells to build enormous molecular diversity: different amino acid sequences generate different polypeptides, which can later fold and function in distinct ways.
Key Takeaways for AP Biology
Peptide bonds are covalent and form specifically between the carboxyl group of one amino acid and the amine group of another.
Formation involves removal of H and OH to release water, producing a linear polypeptide chain with an N-terminus and C-terminus.
FAQ
Cells use enzyme-driven processes to make peptide bonds efficiently and accurately. In vivo, bond formation is coupled to energy-releasing steps rather than occurring freely in solution.
An amide is a functional group with a carbonyl carbon bonded to a nitrogen. In a peptide bond, the linkage is –C(=O)–NH–, which is why peptide bonds are classified as amide bonds.
Both are amino acid chains linked by peptide bonds. “Peptide” often refers to shorter chains, while “polypeptide” usually refers to longer chains, but there is no single universal cut-off.
Because only one end retains a free amino group (N-terminus) and the other retains a free carboxyl group (C-terminus). This asymmetry arises from how peptide bonds link carboxyl-to-amino each time.
Yes. Breaking a peptide bond requires adding the elements of water across the bond (a hydrolytic cleavage). In cells, this is typically catalysed by specific proteases rather than occurring rapidly on its own.
Practice Questions
Explain which two functional groups react to form a peptide bond. (2 marks)
Identifies the carboxyl group of one amino acid (1)
Identifies the amino/amine group of another amino acid (1)
Describe how a peptide bond forms between two amino acids and explain how repeated bonding produces a linear polypeptide chain with two different ends. (6 marks)
States a peptide bond is a covalent bond formed between amino acids (1)
Describes reaction between carboxyl group of one amino acid and amino/amine group of another (1)
Mentions removal of OH from carboxyl and H from amino/amine (1)
Mentions water is produced (condensation/dehydration) (1)
Explains repeated peptide bond formation links many amino acids into a linear/unbranched chain (1)
Identifies that the chain has an N-terminus (free amino group) and a C-terminus (free carboxyl group) (1)

