AP Syllabus focus:
‘Explain how interactions among multiple polypeptide chains create quaternary structure and how all four structural levels determine protein function.’Proteins often work as multi-part assemblies rather than single polypeptide chains. Quaternary structure explains how separate subunits fit and cooperate, and why changes at any structural level can alter overall protein function.
What Quaternary Structure Is
Quaternary structure forms when two or more polypeptide chains (subunits) associate into one functional protein complex.
Quaternary structure: the three-dimensional arrangement and interactions of multiple polypeptide subunits within a single functional protein.
Quaternary structure is not “more folding” of one chain; it is assembly of multiple chains into a higher-order unit.
Subunits and Stoichiometry
A protein complex is defined by:
Number of subunits (e.g., dimer, trimer, tetramer)
Types of subunits (identical vs different)
Stoichiometry: the ratio of subunits (e.g., 2α:2β in a tetramer)
Subunit: an individual polypeptide chain that associates with other chains to form a functional multi-chain protein.
Interactions That Create and Stabilise Quaternary Structure
Subunits are held together primarily by the same categories of forces that stabilise tertiary structure, but between polypeptides rather than within one chain:
Hydrophobic interactions: nonpolar side chains cluster away from water at subunit interfaces, often the dominant stabilising force.
Hydrogen bonds: form between backbone or side-chain polar groups across the interface, adding specificity to subunit pairing.
Ionic (electrostatic) interactions: attractions between oppositely charged side chains (salt bridges) can stabilise assembly, especially in particular pH ranges.
Disulfide bridges: covalent S–S bonds can link subunits (common in secreted proteins), increasing stability in extracellular conditions.
Because these interactions depend on side-chain chemistry, mutations that change R groups at an interface can disrupt assembly without necessarily unfolding each subunit.
Assembly is Specific, Not Random
Quaternary structure depends on molecular complementarity:
Shape fit between subunits (surface contours)
Matching of polar/charged groups for bonding
Correct orientation to produce an active complex
Incorrect subunit pairing can reduce function or produce non-functional aggregates.
How Quaternary Structure Supports Protein Function
Quaternary structure is strongly favoured when function requires coordination, regulation, or mechanical strength that a single polypeptide cannot easily provide.
Functional Advantages of Multimeric Proteins
Cooperativity: binding of a ligand to one subunit changes the conformation of others, altering their binding affinity.
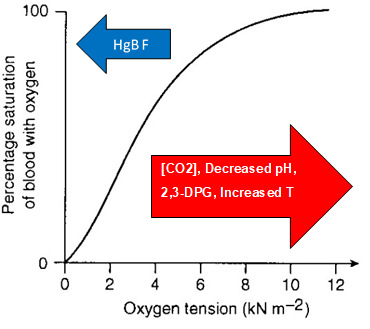
Oxyhemoglobin dissociation curve (sigmoidal binding). This plot shows hemoglobin percent saturation as a function of oxygen partial pressure, producing a characteristic S-shaped curve. The sigmoidal shape reflects cooperative binding: oxygen binding to one subunit shifts other subunits toward a higher-affinity conformation, increasing overall binding at intermediate . Source
Allosteric regulation: binding at one site (often on one subunit) shifts activity at a distant active site on another.
Division of labour: different subunits can have distinct roles (catalytic vs regulatory subunits).
Structural reinforcement: repeating subunits can form stable fibres or scaffolds.
Efficiency and proximity: subunits can position active sites or channels for rapid sequential steps.
Classic examples of quaternary organisation-function relationships include hemoglobin (oxygen-binding tetramer with cooperative behaviour), collagen (multi-chain structural assembly for tensile strength), and antibodies (multiple chains forming antigen-binding sites and constant regions).
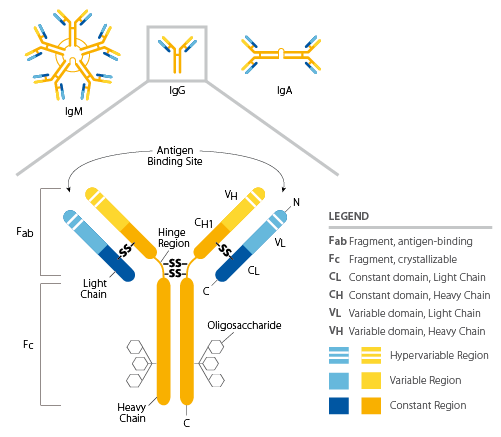
IgG antibody quaternary structure (HL) with disulfide bonds. This diagram shows how two heavy chains and two light chains assemble into a Y-shaped immunoglobulin, with disulfide bridges linking chains to stabilize the complex. It also distinguishes functional regions (antigen-binding arms vs constant region), illustrating how quaternary assembly creates specialized binding and effector functions. Source
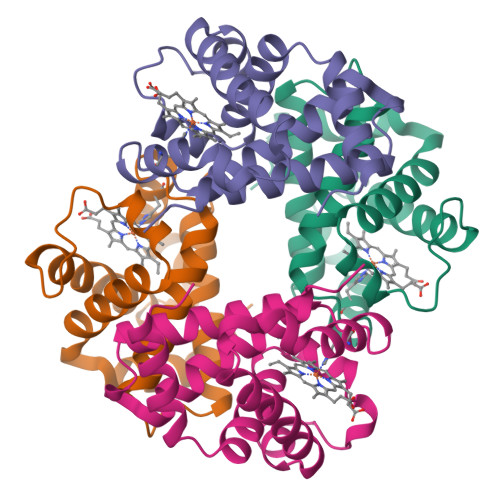
Deoxy human hemoglobin quaternary structure (tetramer). The structure shows four polypeptide subunits assembled into one functional oxygen-transport protein, illustrating how quaternary structure is an arrangement of multiple chains rather than additional folding of a single chain. Hemoglobin’s organization enables subunit–subunit conformational coupling that underlies cooperative oxygen binding. Source
How All Four Structural Levels Determine Protein Function
Protein function reflects an integrated hierarchy; changes at any level can propagate to quaternary assembly and activity.
Primary structure (amino acid sequence) determines which interactions are possible at subunit interfaces; even one substitution can disrupt binding or regulation.
Secondary structure contributes recurring shapes (helices, sheets) that help form stable interface surfaces.
Tertiary structure creates each subunit’s overall 3D shape, forming active sites and the precise interface geometry needed for assembly.
Quaternary structure sets the final arrangement of subunits, enabling cooperativity, allostery, or multi-part active sites.
Thus, quaternary structure both depends on the first three levels and can create new functional properties that emerge only when subunits interact.
FAQ
Common approaches include X-ray crystallography and cryo-electron microscopy to visualise subunit arrangement.
Solution-based methods help confirm subunit number and interfaces, such as native gel electrophoresis and analytical ultracentrifugation.
Obligate complexes have subunits that are usually inseparable under physiological conditions and function only when assembled.
Transient complexes form and dissociate as part of regulation, signalling, or substrate transfer, often controlled by ligand binding or modification.
Repeating the same subunit can be genetically efficient and allows symmetric structures.
Homomers can also enable cooperativity because identical interfaces can transmit conformational changes between subunits.
Modifications can alter charge or shape at subunit interfaces, shifting assembly strength.
Examples include phosphorylation changing electrostatic interactions, or formation/rearrangement of disulfide bonds affecting extracellular stability.
Chaperones can prevent incorrect subunit interactions and aggregation during assembly.
Some complexes require dedicated assembly factors that guide subunits into the correct stoichiometry and orientation before release into the cell.
Practice Questions
State what is meant by quaternary structure in proteins. (2 marks)
Mentions that it involves more than one polypeptide chain/subunit (1)
States that it is the arrangement/interactions of these subunits to form a functional protein (1)
Explain how interactions among multiple polypeptide chains can produce a functional quaternary structure, and describe how disruption at different structural levels could change protein function. (6 marks)
Describes that subunits associate to form one functional complex (1)
Explains stabilising interactions between subunits (any two: hydrophobic, hydrogen bonds, ionic interactions, disulfide bridges) (2)
Links quaternary structure to a functional advantage such as cooperativity/allostery/division of labour/structural strength (1)
Explains how a primary structure change (mutation) can alter interface R groups and disrupt assembly or regulation (1)
Explains how altered tertiary/secondary structure can change subunit shape and prevent correct fit, changing function (1)

