OCR Specification focus:
‘Recognise endocrine tissues of the pancreas from stained sections and relate histology to function.’
The pancreas is a vital organ with both endocrine and exocrine functions, playing a key role in regulating blood glucose levels and digestion through its distinctive histological organisation.
The Dual Role of the Pancreas
The pancreas functions as both an exocrine gland, secreting digestive enzymes into the duodenum, and an endocrine gland, releasing hormones directly into the bloodstream. This duality is evident in its histological structure, with distinct tissue regions performing these complementary roles.
Exocrine Tissue
The exocrine pancreas constitutes the majority of pancreatic tissue. It is made up of numerous acini (singular: acinus), which are small clusters of secretory cells surrounding a ductule that drains into the pancreatic duct.
Acinar cells produce and secrete digestive enzymes, including amylase, lipase, and proteases such as trypsinogen.
These enzymes are stored temporarily in zymogen granules before secretion.
Centroacinar cells and duct cells within the acinar system secrete bicarbonate ions (HCO₃⁻) to neutralise stomach acid entering the duodenum.
The exocrine cells are highly basophilic at their bases (due to abundant rough endoplasmic reticulum for enzyme synthesis) and eosinophilic at their apices (due to zymogen granules), giving a distinctive staining pattern under the microscope.
Endocrine Tissue
Scattered within the exocrine matrix are clusters of endocrine cells known as the islets of Langerhans, which make up about 1–2% of pancreatic tissue but play a crucial role in homeostatic control of blood glucose.
Islets of Langerhans: Clusters of endocrine cells in the pancreas that secrete hormones regulating blood glucose concentration.
Each islet is richly supplied with capillaries, enabling rapid hormone diffusion into the bloodstream. The islets contain several types of hormone-secreting cells distinguishable by staining and function.
Histological Features of the Islets of Langerhans
Cell Types and Their Hormones
The islets are composed of several key cell types, each with a distinct role in glucose regulation:
α (alpha) cells – secrete glucagon, which increases blood glucose concentration by promoting glycogenolysis (breakdown of glycogen to glucose) and gluconeogenesis (formation of glucose from non-carbohydrate sources).
β (beta) cells – secrete insulin, which lowers blood glucose by enhancing cellular uptake of glucose and stimulating glycogenesis (conversion of glucose to glycogen).
δ (delta) cells – release somatostatin, which inhibits secretion of both insulin and glucagon, maintaining balance within the islet microenvironment.
PP (pancreatic polypeptide) cells – produce pancreatic polypeptide, which regulates pancreatic enzyme secretion and gastrointestinal activity.
In histological sections stained with haematoxylin and eosin (H&E) or specific immunostains, these cell types can often be distinguished by their size, location, and staining properties:
β cells are typically more numerous and located centrally within the islet.
α cells are smaller, less abundant, and tend to occupy the periphery.
δ and PP cells are scattered irregularly.
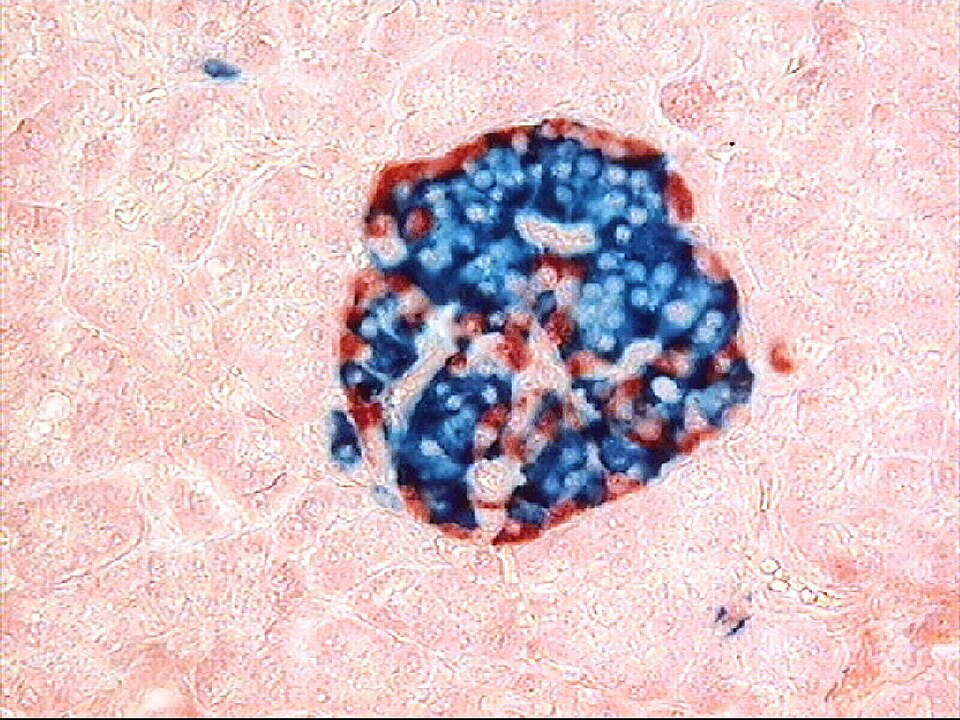
Human islet visualised by double immunostaining: glucagon in red and insulin in blue. This highlights the distribution of α- and β-cells within the islet, complementing routine H&E recognition. Extra detail: Immunofluorescent colours and precise antigen mapping exceed routine OCR requirements but are pedagogically useful for relating histology to function. Source.
Vascularisation and Innervation
The islets have a rich capillary network, allowing direct secretion of hormones into the bloodstream for rapid systemic distribution. Each islet receives afferent arterioles that form fenestrated capillaries, facilitating efficient hormone exchange.
Nerve fibres from the autonomic nervous system modulate hormonal release:
Parasympathetic stimulation enhances insulin secretion.
Sympathetic stimulation promotes glucagon release.
This neural input enables fine-tuned regulation of blood glucose during rest and stress conditions.
Relating Histology to Function
The Endocrine–Exocrine Relationship
The arrangement of exocrine acini interspersed with endocrine islets allows the pancreas to function as a hub of both digestion and homeostasis.
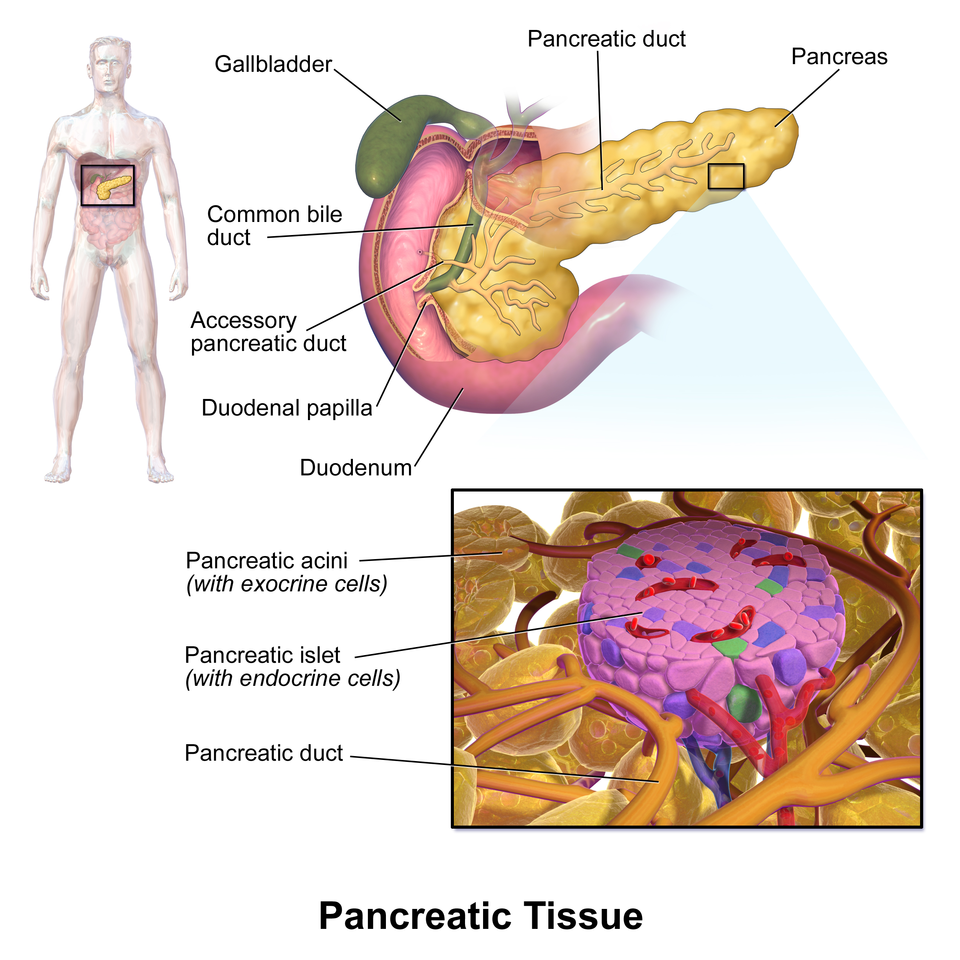
Labeled schematic of pancreatic tissue showing a pale islet of Langerhans within darker exocrine acini, with nearby duct and vessels. This clarifies how endocrine and exocrine compartments sit together to coordinate metabolic and digestive functions. The diagram includes minimal, well-placed labels for rapid identification. Source.
Structural Adaptations for Function
The structural features of the pancreas are tightly linked to its physiological functions:
The abundant rough endoplasmic reticulum in acinar cells supports rapid synthesis of enzyme proteins.
The granular cytoplasm and secretory vesicles reflect active exocytosis.
The capillary-rich islets support immediate hormone release into the bloodstream.
The close proximity of different cell types ensures hormonal feedback coordination — for example, insulin and glucagon counter-regulation.
Microscopy Identification
When examining stained pancreatic sections, OCR students should be able to distinguish:
Exocrine tissue: densely packed acini, with small lumen and strong staining contrast between basophilic bases and eosinophilic apices.
Endocrine tissue (islets): lighter-stained, spherical clusters scattered irregularly among the darker exocrine tissue.
A well-prepared slide may also show interlobular ducts, blood vessels, and occasional connective tissue septa separating lobules.
Functional Importance in Homeostasis
The pancreatic endocrine system exemplifies negative feedback control:
A rise in blood glucose after a meal stimulates β cells to secrete insulin, promoting glucose uptake and storage.
A fall in blood glucose triggers α cells to secrete glucagon, promoting glucose release from stores.
This dynamic equilibrium maintains blood glucose concentration within a narrow physiological range (~4–6 mmol dm⁻³).
Disruption of these processes can lead to metabolic disorders, such as diabetes mellitus, characterised by defective insulin production or response, though detailed study of such conditions belongs to later subsubtopics.
Integration with Other Systems
The pancreas interacts closely with the liver and adrenal glands in maintaining energy balance. Hormones like adrenaline can influence pancreatic hormone release, illustrating the body’s integrated endocrine network.
Through its intricate histological design, the pancreas demonstrates how form and function converge to achieve precise homeostatic regulation across multiple systems.
FAQ
The islets of Langerhans originate from the same endodermal tissue that forms the exocrine pancreas. During development, clusters of undifferentiated pancreatic cells migrate and specialise into endocrine cells.
Beta cells are usually the first to mature, followed by alpha and delta cells. The arrangement of these cells into distinct clusters occurs alongside the development of the islet’s capillary network, which ensures efficient hormone delivery once the pancreas becomes functional after birth.
Beta cells make up about 60–80% of the islet’s cell population because they produce insulin, which is continuously needed to regulate blood glucose following food intake.
In contrast, alpha cells (15–20%) mainly function during fasting or energy shortage. The higher number of beta cells allows precise control of glucose uptake and storage, ensuring small fluctuations in glucose levels can be corrected rapidly.
The islets contain fenestrated capillaries, which have pores in their endothelial walls.
These openings allow rapid exchange of hormones such as insulin and glucagon between the endocrine cells and the bloodstream.
This structure ensures that hormone release is immediate and that glucose regulation occurs efficiently.
The close proximity of endocrine cells to capillaries also facilitates paracrine signalling between cell types within the same islet.
Although the two regions function differently, they are coordinated by both nervous and hormonal signals.
Parasympathetic stimulation enhances both insulin secretion and enzyme release during digestion.
Somatostatin, released by delta cells, can locally inhibit both endocrine and exocrine activity, preventing over-secretion.
Shared blood flow between islets and acinar tissue ensures that hormonal changes can rapidly influence digestive processes.
This coordination enables the pancreas to synchronise metabolic regulation with digestion.
Immunohistochemistry uses antibodies tagged with coloured or fluorescent markers to bind specific hormones within pancreatic cells.
Anti-insulin antibodies identify beta cells.
Anti-glucagon antibodies reveal alpha cells.
Anti-somatostatin antibodies mark delta cells.
These stains allow researchers and pathologists to map the cellular composition of islets accurately. They are particularly valuable for studying diabetes, as they reveal beta-cell loss or dysfunction that cannot be seen in standard H&E sections.
Practice Questions
Question 1 (2 marks)
Describe the difference in function between the exocrine and endocrine tissues of the pancreas.
Mark scheme:
1 mark for stating that exocrine tissue secretes digestive enzymes into ducts or the duodenum.
1 mark for stating that endocrine tissue secretes hormones (such as insulin and glucagon) directly into the bloodstream.
Question 2 (5 marks)
The islets of Langerhans are visible in stained sections of the pancreas as pale-staining clusters surrounded by darker exocrine tissue.
Explain how the structural features of the islets of Langerhans relate to their role in maintaining blood glucose concentration.
Mark scheme:
1 mark for identifying that the islets contain different types of hormone-secreting cells (e.g. alpha and beta cells).
1 mark for stating that beta cells secrete insulin, which lowers blood glucose by promoting glucose uptake and glycogenesis.
1 mark for stating that alpha cells secrete glucagon, which raises blood glucose by promoting glycogenolysis and gluconeogenesis.
1 mark for describing that the islets have a rich capillary network, allowing rapid hormone release into the bloodstream.
1 mark for explaining that these features enable negative feedback control of blood glucose concentration, maintaining homeostasis.

