OCR Specification focus:
‘Contrast causes of Type 1 and Type 2 diabetes and outline treatments used for each condition.’
Diabetes mellitus is a chronic disorder of blood glucose regulation, caused by defects in insulin secretion or response. Type 1 and Type 2 diabetes differ in causes, onset, and management.
Understanding Diabetes Mellitus
Diabetes mellitus refers to conditions where blood glucose concentration remains persistently above the normal range due to a failure in the regulation of insulin production or action. Insulin, a hormone secreted by beta cells in the islets of Langerhans in the pancreas, lowers blood glucose by promoting uptake into cells and stimulating glycogen formation.
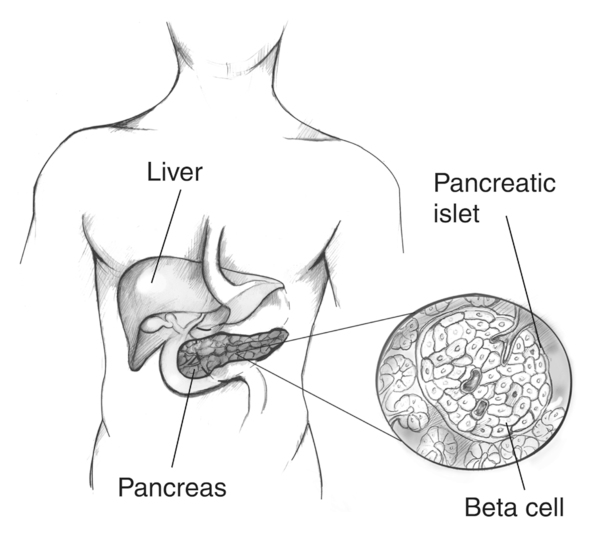
Labeled illustration of the pancreas with an enlarged islet of Langerhans highlighting beta cells, which produce and release insulin into the blood. This visual clarifies the cellular target of autoimmune destruction in Type 1 diabetes and the source of insulin dysregulation in Type 2. Clean labels match syllabus terminology; no extraneous pathways are shown. Source.
When this regulation fails, hyperglycaemia (excess blood glucose) occurs, leading to symptoms such as excessive thirst (polydipsia), frequent urination (polyuria), fatigue, and weight loss.
Insulin: A peptide hormone produced by pancreatic beta cells that lowers blood glucose by promoting glucose uptake and glycogen synthesis.
Insulin’s essential role in maintaining homeostasis makes defects in its production or function particularly harmful.
Type 1 Diabetes Mellitus
Overview
Type 1 diabetes mellitus (T1DM) is an autoimmune condition where the body’s immune system destroys the insulin-producing beta cells of the pancreas. This results in an absolute deficiency of insulin.
Key Features
Usually develops in childhood or adolescence.
Rapid onset of symptoms due to complete loss of insulin secretion.
Requires lifelong insulin therapy for survival.
Accounts for around 10% of all diabetes cases.
Causes
The exact trigger for autoimmunity is not fully understood but is believed to involve:
Genetic predisposition – inheritance of certain alleles in the HLA (human leukocyte antigen) region on chromosome 6.
Environmental triggers – such as viral infections (e.g. Coxsackie virus).
Autoimmune response – T lymphocytes mistakenly attack beta cells, resulting in their destruction and inability to secrete insulin.
Pathophysiology
Without insulin:
Glucose cannot enter cells, remaining in the bloodstream.
The body relies on fat and protein breakdown for energy, leading to weight loss and ketone accumulation.
Excessive ketone bodies cause diabetic ketoacidosis (DKA), a potentially fatal condition due to metabolic acidosis.
Treatment
Insulin injections or insulin pumps must be administered regularly to maintain normal blood glucose.
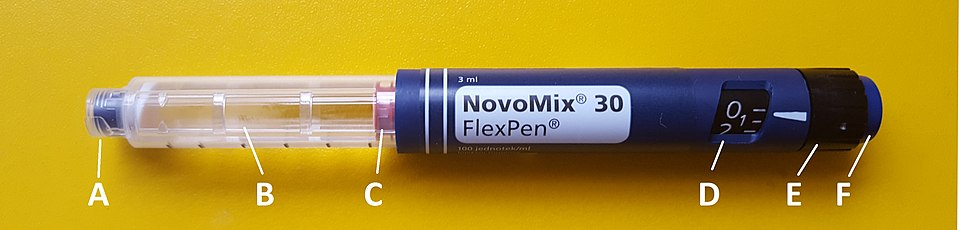
Photograph of an insulin pen with labelled parts (tip, medication chamber, plunger, dose window, selection dial, plunger button). This clarifies practical delivery of exogenous insulin for Type 1 diabetes. The device anatomy exceeds the bare syllabus but remains concise and educational. Source.
Monitoring blood glucose levels using digital glucometers is essential.
Dietary management supports stable glucose levels, but insulin replacement remains the central therapy.
Type 2 Diabetes Mellitus
Overview
Type 2 diabetes mellitus (T2DM) is characterised by insulin resistance and impaired insulin secretion. In this form, the pancreas still produces insulin, but the body’s target cells respond less effectively to it.
Key Features
Commonly develops in adulthood, though increasingly seen in younger individuals due to lifestyle factors.
Has a gradual onset.
Initially managed by lifestyle changes and oral medication, progressing to insulin therapy if beta cell function declines.
Represents around 90% of all diabetes cases.
Causes
Type 2 diabetes is multifactorial, involving both genetic and environmental influences:
Genetic predisposition – strong familial link; several genes affect insulin receptor sensitivity and beta cell function.
Lifestyle factors – obesity, physical inactivity, and high-calorie diets increase risk.
Age-related decline – reduced pancreatic responsiveness and tissue sensitivity to insulin over time.
Pathophysiology
Insulin resistance in muscle, liver, and adipose tissue reduces glucose uptake and increases hepatic glucose output.
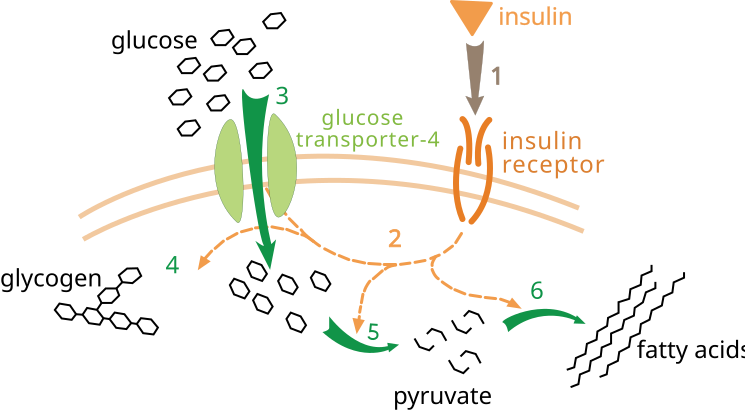
Simplified diagram of insulin binding its receptor, triggering signalling that moves GLUT-4 to the cell surface to permit glucose entry. This mechanism underlies why insulin resistance diminishes uptake in Type 2 diabetes. The image also labels downstream fates (glycogen, glycolysis, fatty acid synthesis); these are helpful extensions beyond the syllabus core. Source.
Beta cells initially compensate by producing more insulin, but chronic overwork leads to beta cell exhaustion.
Blood glucose rises progressively, resulting in chronic hyperglycaemia.
Treatment
Lifestyle modification is first-line therapy:
Regular exercise increases insulin sensitivity.
Weight loss can restore normal glucose tolerance in some cases.
Balanced diet reduces postprandial glucose spikes.
Oral hypoglycaemic agents:
Metformin reduces hepatic glucose production and increases insulin sensitivity.
Sulphonylureas stimulate insulin secretion by beta cells.
DPP-4 inhibitors and GLP-1 analogues enhance insulin release and suppress glucagon.
Insulin injections may be required in advanced stages when beta cells fail.
Comparing Type 1 and Type 2 Diabetes
Aetiology
Type 1: Autoimmune destruction of beta cells leading to total insulin deficiency.
Type 2: Insulin resistance and relative insulin deficiency due to beta cell fatigue.
Onset and Demographics
Type 1: Sudden onset, usually in childhood or adolescence.
Type 2: Gradual onset, typically in adults over 40 but increasingly common in younger age groups.
Insulin Production
Type 1: None; external insulin is essential.
Type 2: Initially normal or high, later declining due to beta cell failure.
Reversibility and Prevention
Type 1: Not preventable or reversible.
Type 2: Often preventable and may be reversed in early stages through lifestyle change.
Insulin resistance: A condition where target cells (e.g. muscle, liver, adipose) fail to respond adequately to normal insulin levels, reducing glucose uptake.
Chronic insulin resistance drives metabolic disturbances affecting the cardiovascular and renal systems.
Complications and Long-Term Effects
Both types can cause chronic complications if poorly managed:
Microvascular damage: retinopathy (eye), nephropathy (kidneys), and neuropathy (nerves).
Macrovascular disease: atherosclerosis, increasing risk of stroke and heart disease.
Poor wound healing and increased infection risk due to reduced immune efficiency.
Monitoring and Management
To prevent these:
Maintain blood glucose within normal range (approximately 4–7 mmol dm⁻³).
Regular HbA1c testing provides long-term glucose control data.
Continuous education and support promote adherence to treatment.
Modern Therapeutic Developments
Research continues into new treatment methods:
Islet cell transplantation for Type 1 patients shows potential for restoring endogenous insulin production.
Artificial pancreas systems integrate glucose sensors and insulin pumps for automatic regulation.
Lifestyle intervention programmes remain crucial for reducing the growing prevalence of Type 2 diabetes worldwide.
FAQ
The exact cause remains unclear, but it’s thought to result from a combination of genetic and environmental factors.
Genetic susceptibility involves certain HLA genes that make the immune system more likely to recognise beta cells as foreign. Environmental triggers such as viral infections (e.g. enteroviruses), early exposure to cow’s milk proteins, or vitamin D deficiency may initiate the immune response.
Once activated, T lymphocytes target and destroy pancreatic beta cells, leading to complete insulin deficiency.
Excess body fat, especially around the abdomen, leads to chronic low-grade inflammation and the release of substances known as adipokines.
These interfere with insulin signalling in target tissues like muscle and liver, making them insulin resistant. As a result, cells fail to take up glucose effectively, and blood glucose levels rise.
Over time, the pancreas compensates by secreting more insulin, but beta cells eventually become exhausted, reducing insulin output and causing persistent hyperglycaemia.
The liver regulates blood glucose concentration through glycogenesis, glycogenolysis, and gluconeogenesis.
In healthy individuals, insulin promotes glycogenesis, storing glucose as glycogen.
In diabetes, reduced insulin action allows uncontrolled gluconeogenesis, increasing glucose release into the bloodstream.
The liver also contributes to lipid metabolism changes, leading to higher levels of triglycerides and fatty acids commonly seen in diabetic patients.
This disruption exacerbates hyperglycaemia and contributes to metabolic complications.
Prolonged hyperglycaemia leads to glycation, where glucose binds to proteins in blood vessel walls, making them thick and less elastic.
This process triggers oxidative stress and inflammation.
Small vessels (microvasculature) in the eyes, kidneys, and nerves become damaged, leading to retinopathy, nephropathy, and neuropathy.
Larger arteries (macrovasculature) are prone to atherosclerosis, increasing the risk of heart disease and stroke.
Good long-term glucose control (measured by HbA1c) helps prevent or slow these complications.
In early Type 2 diabetes, the pancreas still produces insulin, but it’s less effective due to resistance in target cells.
Over time, continuous overproduction of insulin causes beta cell fatigue and a decline in their function.
When oral medications and lifestyle interventions can no longer maintain normal glucose levels, insulin therapy is introduced to supplement the body’s declining supply and restore better glycaemic control.
Practice Questions
Question 1 (2 marks)
Explain why insulin injections are required for individuals with Type 1 diabetes but not always for those with Type 2 diabetes.
Mark Scheme:
Type 1 diabetes results from destruction of pancreatic beta cells by the immune system (1)
Therefore, no insulin is produced and it must be supplied externally via injections (1)
(Reject answers that state Type 2 individuals never use insulin; accept that insulin may be used later when beta cell function declines)
Question 2 (5 marks)
Compare and contrast Type 1 and Type 2 diabetes in terms of their causes, onset, and typical treatments.
Mark Scheme:
Award 1 mark for each relevant comparison up to 5 marks:
Cause: Type 1 is autoimmune, destroying beta cells; Type 2 results from insulin resistance and reduced responsiveness of target cells (1)
Insulin production: Absent in Type 1; initially present but less effective in Type 2 (1)
Onset: Type 1 has sudden onset often in childhood/adolescence; Type 2 develops gradually, typically in adulthood (1)
Treatment: Type 1 requires insulin injections for life; Type 2 managed by diet, exercise, and oral medication, with insulin used later if needed (1)
Prevention/reversibility: Type 1 cannot be prevented; Type 2 is often linked to lifestyle and can be prevented or reversed through lifestyle changes (1)

