OCR Specification focus:
‘Outline insulin produced by genetically modified bacteria and the potential use of stem cells to treat diabetes mellitus.’
Treatments for diabetes have advanced from regular insulin injections to modern biotechnological methods using genetically modified bacteria and stem cell therapy, improving disease management and patient outcomes.
Insulin Production by Genetically Modified Bacteria
Historical Context
Before genetic engineering, insulin used for Type 1 diabetes treatment was extracted from the pancreas of pigs and cows. While this method saved lives, it had several drawbacks:
Animal insulin had slight structural differences from human insulin, sometimes triggering immune reactions.
Supply relied on slaughterhouse by-products, making production limited and inconsistent.
Religious or ethical concerns arose regarding the use of animal tissues.
Biotechnology solved these problems by enabling large-scale synthesis of human insulin through genetically modified (GM) bacteria.
Genetic Engineering Process
The production of human insulin using recombinant DNA technology involves inserting the human insulin gene into bacterial plasmids, allowing the bacteria to synthesise insulin identical to the human form.
Steps in Insulin Production:
Isolation of the insulin gene:
Scientists identify and copy the gene coding for human insulin from a human cell.
Insertion into a plasmid vector:
The plasmid, a small circular DNA molecule in bacteria, is cut using restriction enzymes.
The human insulin gene is inserted using DNA ligase to form recombinant DNA.
Transformation of host bacteria:
The recombinant plasmid is introduced into Escherichia coli (E. coli) cells.
Expression of the insulin gene:
The bacterial cells use their cellular machinery to transcribe and translate the human insulin gene, producing proinsulin.
Purification and processing:
Proinsulin is extracted and modified to form functional human insulin before packaging for medical use.
Advantages of GM Insulin
Genetically engineered insulin offers significant improvements over older methods:
Identical to human insulin, eliminating immune rejection.
Reliable and scalable production, ensuring stable global supply.
Ethically acceptable, as it avoids animal sources.
Rapid onset and flexible formulations, such as short-acting and long-acting insulins tailored to patient needs.
Recombinant DNA Technology: The manipulation of DNA to include genetic material from different species, enabling organisms like bacteria to produce useful human proteins such as insulin.
Commercial and Ethical Considerations
Pharmaceutical companies such as Eli Lilly and Novo Nordisk have developed insulin from GM bacteria since the 1980s, making it a global standard. However, intellectual property and cost remain barriers in developing countries.
Despite these issues, recombinant insulin represents one of the greatest successes of medical biotechnology, offering consistent quality and reduced side effects.
Potential Use of Stem Cells in Treating Diabetes
Rationale for Stem Cell Therapy
Type 1 diabetes mellitus results from the autoimmune destruction of pancreatic β (beta) cells, which normally secrete insulin. Current insulin therapies manage but do not cure the disease. Stem cell therapy aims to replace lost β-cells, restoring the body’s natural glucose regulation.
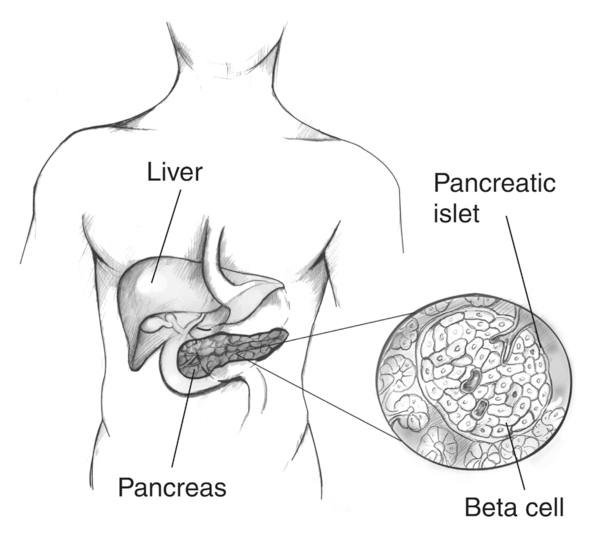
A labeled diagram locates the pancreas and zooms into an islet of Langerhans, highlighting β-cells that secrete insulin. This clarifies the cellular target of replacement strategies discussed in the notes. The image includes only essential labels relevant to the syllabus. Source.
Stem Cells: Undifferentiated cells capable of dividing and specialising into various cell types, offering potential to regenerate damaged tissues.
Sources of Stem Cells
Stem cells used in research and therapy may come from:
Embryonic stem cells (ESCs) — pluripotent and can differentiate into any cell type, including β-cells.
Adult stem cells — found in tissues like bone marrow or liver; more limited in potential.
Induced pluripotent stem cells (iPSCs) — adult cells reprogrammed to behave like embryonic stem cells without using embryos.
Each type has benefits and challenges related to availability, ethical acceptability, and differentiation efficiency.
Differentiation into β-Cells
Scientists use specific chemical signals and growth factors to direct stem cells to become pancreatic β-like cells capable of secreting insulin in response to glucose levels.
Stages of Differentiation:
Induce cells to form endodermal tissue (the embryonic layer that produces pancreatic organs).
Guide development into pancreatic progenitor cells.
Mature these cells into functional insulin-secreting β-cells.
Once transplanted, these cells could potentially restore endogenous insulin production, reducing or eliminating the need for injections.
Challenges and Risks
Although promising, stem cell treatment for diabetes faces several scientific and clinical obstacles:
Immune rejection: The body may attack new β-cells, especially in autoimmune Type 1 diabetes.
Tumour formation: Undifferentiated stem cells can divide uncontrollably, creating risks of cancer.
Ethical issues: The use of embryonic stem cells raises moral and religious concerns.
Regulatory approval: Long-term clinical trials are required to confirm safety and effectiveness.
Current Developments
Recent advances have shown that stem-cell derived pancreatic cells can respond to blood glucose levels and secrete insulin in animal and early human trials. Researchers are exploring encapsulation technologies, in which β-cells are enclosed within semi-permeable membranes to protect them from immune attack while allowing nutrient and hormone exchange.
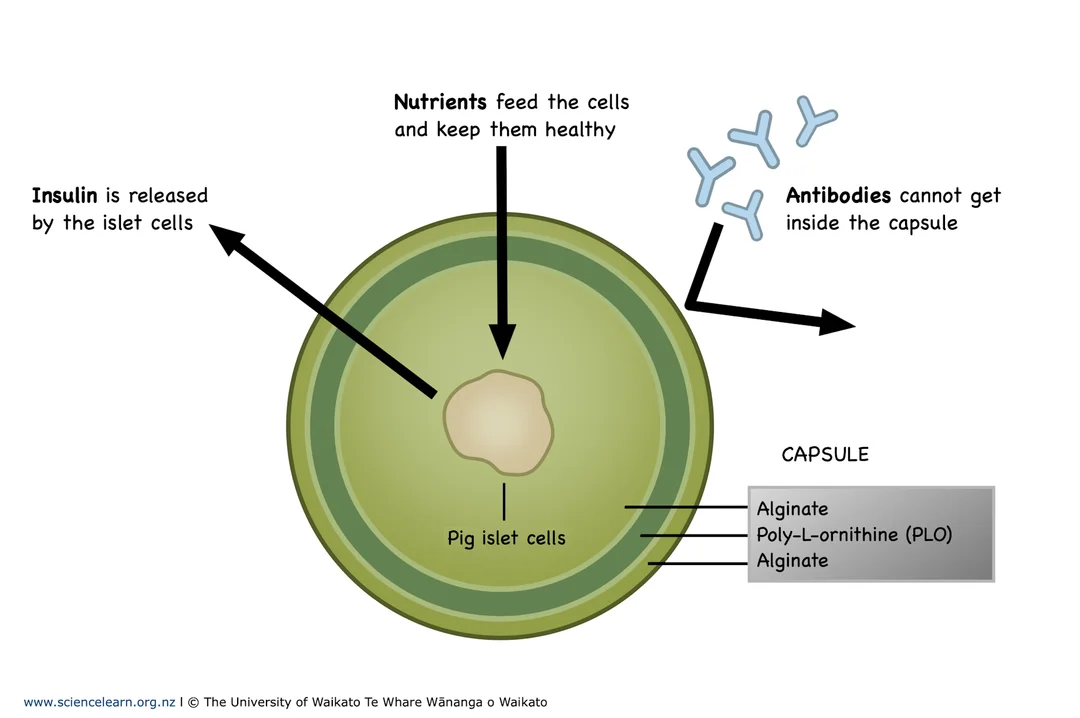
A simplified diagram shows islet cells surrounded by a semi-permeable alginate capsule that permits diffusion of nutrients, oxygen, glucose and insulin while limiting antibody access. This captures the essential engineering idea referenced in the notes. Extra detail: the example depicts pig (porcine) islets to illustrate the same encapsulation principle used for donor or stem-cell-derived human cells. Source.
Ongoing Research Focus:
Developing iPSC-based therapies to avoid ethical issues and immune rejection.
Refining cell differentiation protocols to increase insulin secretion efficiency.
Investigating gene editing (CRISPR-Cas9) to enhance β-cell survival and function.
Long-Term Potential
If perfected, stem cell therapy could provide a functional cure for Type 1 diabetes by restoring physiological glucose regulation. Combined with immunomodulation or gene therapy, such treatments could permanently replace lifelong insulin dependence.
Integrating Modern Approaches
The development of biotechnological insulin and stem cell-based therapies illustrates the shift from symptom management to biological restoration. These innovations exemplify homeostatic regulation at a molecular level—restoring normal glucose balance through targeted biological engineering rather than external compensation.
By harnessing recombinant DNA technology and cellular regeneration, modern medicine continues to expand the possibilities for curing metabolic diseases like diabetes mellitus rather than merely managing them.
FAQ
After E. coli produce proinsulin, the protein is separated from bacterial components using filtration and chromatography.
Affinity chromatography isolates insulin using a column that binds specifically to the insulin molecule.
The proinsulin chain is then chemically treated to form the active A and B chains joined by disulphide bonds.
Final purification removes any contaminants or bacterial remnants to ensure pharmaceutical-grade purity suitable for human use.
This multi-step process ensures the insulin is chemically identical to that produced by human pancreatic β-cells.
Plasmids act as vectors, carrying the human insulin gene into bacterial cells. Their usefulness arises from:
Small, circular DNA structure that can be easily manipulated in the lab.
The presence of replication origins, allowing them to replicate independently within bacterial cells.
Selectable markers (like antibiotic resistance genes) make it easy to identify successfully modified bacteria.
Their versatility and stability make plasmids an ideal vehicle for recombinant DNA technology.
The main ethical debate concerns embryonic stem cells (ESCs), which are derived from early embryos. Removing ESCs destroys the embryo, raising moral and religious concerns about the status of embryonic life.
Alternatives such as induced pluripotent stem cells (iPSCs) avoid this issue by reprogramming adult cells to behave like embryonic ones. iPSCs reduce ethical conflict while still offering pluripotency, making them a preferred option in many countries.
Ethical approval and transparency are required before any clinical use.
Researchers use several strategies:
Encapsulation: placing β-cells inside semi-permeable capsules that allow insulin and nutrients through but block immune cells.
Gene editing: removing or modifying genes responsible for immune recognition (e.g., HLA markers).
Immunosuppressive drugs: temporarily dampening the immune system during cell transplantation.
Encapsulation and gene-editing methods are being refined to avoid lifelong reliance on immunosuppressants.
Stem-cell therapy could restore the body’s natural regulation of blood glucose, providing a long-term or even permanent solution for Type 1 diabetes.
Advantages include:
Continuous insulin secretion in response to glucose levels, preventing hyperglycaemia and hypoglycaemia.
Reduced dependence on daily insulin injections and blood monitoring.
Potential for fewer long-term complications, such as kidney or nerve damage.
If proven safe and reliable, this approach could transform diabetes management from treatment to functional cure.
Practice Questions
Question 1 (2 marks)
Explain why insulin produced by genetically modified bacteria is preferred over insulin extracted from animal sources.
Mark Scheme:
1 mark for stating that genetically modified bacteria produce insulin identical to human insulin.
1 mark for stating that it reduces immune reactions or ethical concerns associated with animal-sourced insulin.
Question 2 (5 marks)
Stem cell therapy is being developed as a potential treatment for Type 1 diabetes.
Describe how stem cells could be used to treat this condition and discuss two potential challenges of using this therapy.
Mark Scheme:
1 mark for stating that stem cells can differentiate into pancreatic beta cells.
1 mark for explaining that these beta cells produce and secrete insulin in response to blood glucose levels.
1 mark for describing that the transplanted cells could restore blood glucose regulation, reducing or eliminating the need for insulin injections.
1 mark for identifying a valid challenge, such as immune rejection or tumour formation from uncontrolled cell growth.
1 mark for identifying a second valid challenge, such as ethical issues with embryonic stem cells or the need for long-term safety testing.

