OCR Specification focus:
‘Describe beta-cell control of insulin secretion, involving potassium channels, calcium channels and vesicle exocytosis.’
Insulin secretion from pancreatic beta cells is a vital homeostatic process that ensures blood glucose concentration remains within a narrow physiological range, preventing hyperglycaemia and hypoglycaemia.
The Pancreatic Islets of Langerhans
Within the pancreas, clusters of endocrine tissue called Islets of Langerhans contain two key cell types:
Alpha (α) cells, which secrete glucagon, and
Beta (β) cells, which secrete insulin.
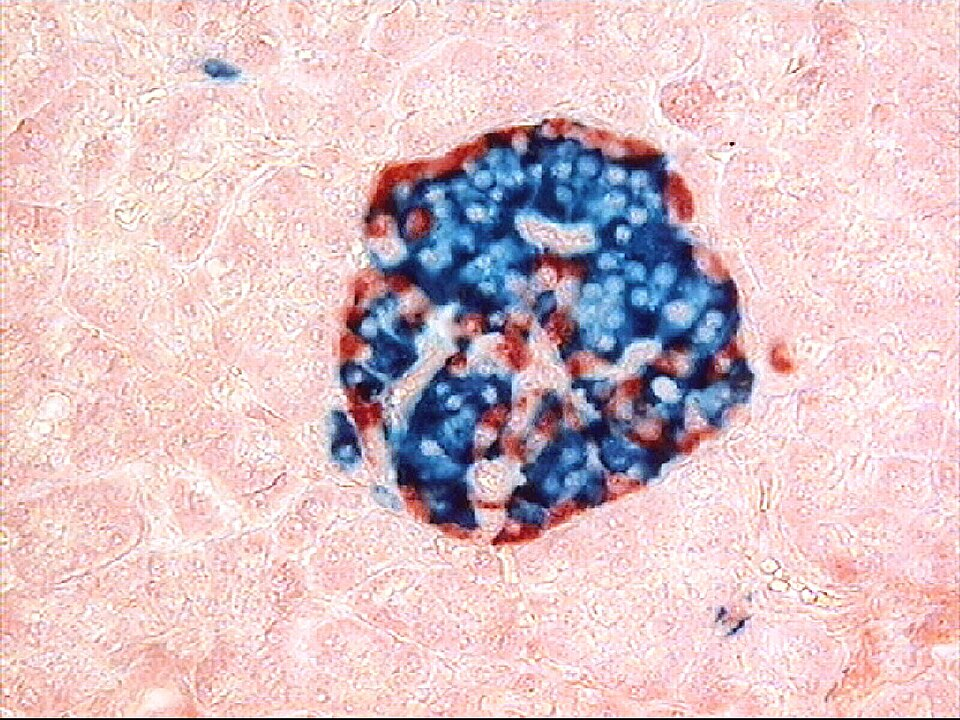
Light micrograph of a human pancreatic islet surrounded by exocrine tissue. The islet contains β cells (insulin-secreting) and α cells (glucagon-secreting), the antagonistic partners in glucose homeostasis. Label density is modest and suitable for OCR level; no extra pathways beyond the specification are shown. Source.
These hormones act antagonistically to regulate blood glucose concentration through negative feedback mechanisms.
Beta cells are highly specialised to sense changes in blood glucose levels and adjust insulin secretion accordingly. Their response is electrical and chemical, linking metabolism with membrane transport and vesicle release.
Glucose Detection and Beta Cell Activation
Glucose Uptake and Metabolism
When blood glucose levels rise after eating, glucose enters beta cells via GLUT2 transporters on the plasma membrane. These transporters are facilitated diffusion channels that allow glucose entry proportional to its concentration in the blood.
Once inside the cell, glucose undergoes aerobic respiration through glycolysis and the Krebs cycle to produce adenosine triphosphate (ATP).
ATP (Adenosine Triphosphate): A high-energy compound that stores and transfers energy within cells for metabolic processes.
The resulting increase in ATP concentration acts as a crucial signal for the next steps of insulin secretion.
Role of Potassium (K⁺) Channels
In the resting state (before glucose uptake), the beta-cell membrane contains ATP-sensitive potassium channels (KATP channels). These channels remain open, allowing potassium ions (K⁺) to diffuse out of the cell, maintaining a negative membrane potential (approximately –70 mV).
When glucose metabolism increases intracellular ATP, the ATP molecules bind to and close the KATP channels. This reduces potassium efflux, causing the inside of the cell to become less negative — a process known as depolarisation of the cell membrane.
Depolarisation: The reduction of the electrical potential difference across a membrane, making the inside of the cell less negative compared to the outside.
Depolarisation is a key electrical signal that triggers subsequent ionic events.
Opening of Voltage-Gated Calcium Channels
The depolarisation of the beta-cell membrane stimulates the opening of voltage-gated calcium (Ca²⁺) channels. Calcium ions, which are at a much higher concentration outside the cell, diffuse into the cytoplasm.
This rapid influx of Ca²⁺ ions acts as a second messenger, initiating a cascade that leads to vesicle exocytosis.
Second messenger: An intracellular signalling molecule that amplifies and transmits a signal from a receptor or ion channel to target processes inside the cell.
The rise in cytoplasmic Ca²⁺ concentration is therefore the direct trigger for insulin release.
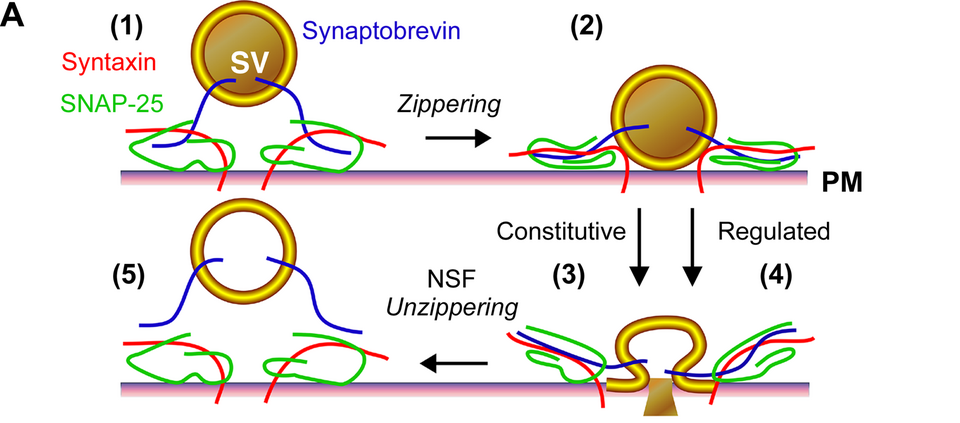
Diagram of SNARE complex assembly driving vesicle–plasma membrane fusion during exocytosis. The figure illustrates docking, zippering and fusion-pore opening, the final step that releases insulin. Protein names reflect general exocytotic machinery; this includes extra detail (e.g. specific SNARE components) beyond the OCR specification but clarifies the mechanism. Source.
Vesicle Exocytosis and Insulin Release
The Role of Vesicles
Insulin is synthesised in the rough endoplasmic reticulum of beta cells as proinsulin, then processed in the Golgi apparatus to form mature insulin. It is stored in secretory vesicles until release is triggered.
When Ca²⁺ levels rise:
Calcium binds to regulatory proteins on the vesicle membranes (e.g. synaptotagmin).
This promotes the fusion of vesicle and plasma membranes through SNARE proteins.
Insulin is released into the bloodstream by exocytosis.
Exocytosis: The process by which substances contained within vesicles are expelled from a cell when the vesicle membrane fuses with the plasma membrane.
The released insulin travels through the bloodstream to target tissues such as liver, muscle, and adipose tissue, promoting glucose uptake and storage.
Summary of the Sequence of Events
The entire mechanism of insulin secretion in beta cells follows this ordered pathway:
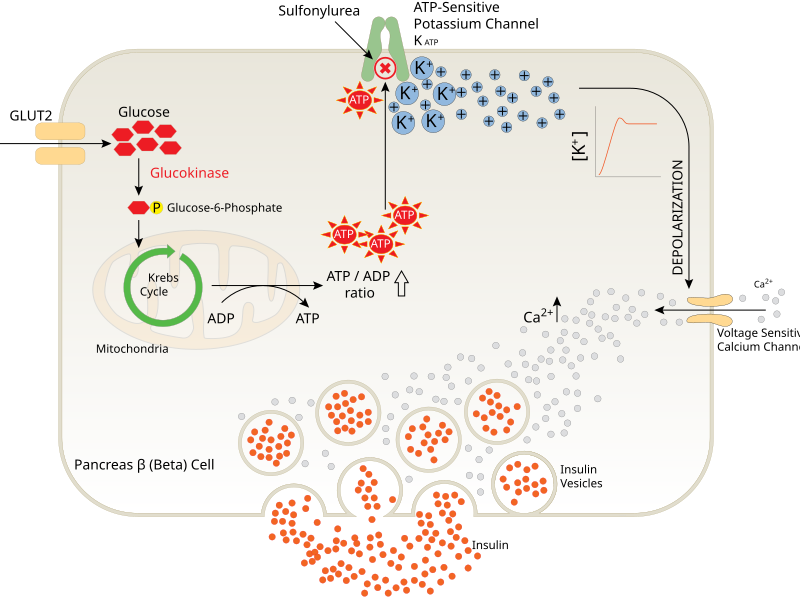
Mechanistic overview of β-cell stimulus–secretion coupling: GLUT2-mediated glucose entry, ATP rise → KATP channel closure, membrane depolarisation → Ca²⁺ influx, and granule exocytosis. The diagram aligns with specification depth; any additional labels serve to orient steps without exceeding OCR scope. As an SVG, it remains sharp at any size. Source.
High blood glucose → increased glucose uptake via GLUT2 transporters.
Glucose metabolism → ATP production increases inside beta cells.
ATP-sensitive K⁺ channels close, preventing potassium efflux.
Membrane depolarises due to accumulation of positive charge.
Voltage-gated Ca²⁺ channels open, allowing Ca²⁺ influx.
Cytoplasmic Ca²⁺ concentration rises.
Insulin-containing vesicles fuse with the plasma membrane.
Insulin is released into the blood via exocytosis.
Each step transforms a metabolic signal (ATP) into an electrical event (depolarisation) and then a secretory response (insulin release).
Modulation and Regulation of Insulin Secretion
Negative Feedback Control
Once insulin promotes glucose uptake into cells and blood glucose concentration returns to normal, less glucose enters the beta cells. ATP production declines, KATP channels reopen, membrane potential is restored, and insulin secretion decreases — a negative feedback loop.
Negative feedback: A control mechanism where a change in a physiological variable triggers a response that counteracts the original change, restoring equilibrium.
This self-regulating system maintains homeostasis of blood glucose levels.
Additional Influences
Other factors can modulate insulin release:
Amino acids and fatty acids can enhance insulin secretion.
Parasympathetic nervous stimulation (via acetylcholine) promotes insulin release during digestion.
Sympathetic stimulation (via adrenaline) inhibits insulin secretion, ensuring glucose remains available during stress or exercise.
Certain hormones, such as glucagon-like peptide-1 (GLP-1), potentiate the insulin response to oral glucose intake.
These interactions demonstrate the integration of hormonal and neural control in maintaining energy balance.
Clinical Relevance
In Type 1 diabetes mellitus, beta cells are destroyed by an autoimmune response, eliminating the ability to produce insulin.
In Type 2 diabetes mellitus, insulin secretion may be impaired, or target tissues become insulin resistant, reducing the hormone’s effectiveness.
Understanding the cellular control of insulin secretion provides insight into how these diseases disrupt homeostasis and how treatments — such as sulphonylurea drugs, which close KATP channels to stimulate insulin release — exploit this mechanism.
FAQ
Beta cells respond dynamically because GLUT2 transporters allow glucose entry at a rate proportional to blood glucose concentration. As intracellular glucose rises, ATP production increases via glycolysis and oxidative phosphorylation. The resulting change in the ATP:ADP ratio directly affects ATP-sensitive potassium (KATP) channels, enabling beta cells to act as natural glucose sensors without separate receptor proteins. This rapid coupling between metabolism and membrane potential ensures accurate insulin release.
ATP-sensitive potassium channels consist of Kir6.2 (pore-forming) and SUR1 (regulatory) subunits.
When ATP binds to Kir6.2, the channel closes, preventing potassium efflux.
When ADP binds to SUR1, the channel opens, promoting potassium flow.
This balance enables precise control of membrane potential, linking cellular energy state directly to electrical activity and insulin secretion.
Beyond initiating vesicle fusion, Ca²⁺ ions modulate intracellular signalling and regulate the rate of exocytosis.
They activate calmodulin and protein kinases, which enhance vesicle movement toward the membrane.
Sustained Ca²⁺ levels also regulate gene transcription for insulin synthesis and vesicle protein replenishment.
Thus, calcium provides both a rapid trigger and a longer-term regulatory signal within beta cells.
Insulin release occurs through a two-phase process:
First phase – pre-docked vesicles fuse rapidly with the membrane, releasing stored insulin.
Second phase – reserve vesicles are mobilised from the cytoplasm for sustained secretion.
The process relies on SNARE proteins (e.g., syntaxin, SNAP-25, synaptobrevin) that ensure vesicle fusion occurs only at the correct site and time, preventing uncontrolled hormone release.
Sulphonylurea drugs (e.g., glibenclamide) bind to SUR1 subunits of ATP-sensitive potassium channels in beta cells.
This binding closes the KATP channels even without a rise in ATP.
The resulting depolarisation opens voltage-gated Ca²⁺ channels, increasing intracellular calcium.
Elevated Ca²⁺ triggers insulin vesicle exocytosis, mimicking natural glucose-stimulated secretion.
This pharmacological effect compensates for reduced beta-cell sensitivity to glucose in early Type 2 diabetes.
Practice Questions
Question 1 (2 marks)
Explain how an increase in blood glucose concentration leads to the depolarisation of the beta-cell membrane in the pancreas.
Mark scheme:
Glucose enters beta cells via GLUT2 transporters. (1 mark)
Glucose metabolism increases ATP concentration, causing ATP-sensitive potassium (KATP) channels to close. (1 mark)
Closing KATP channels prevents potassium efflux, leading to membrane depolarisation. (Accept any two relevant points for 2 marks.)
Question 2 (5 marks)
Describe the sequence of events that occur in a pancreatic beta cell from the initial uptake of glucose to the release of insulin into the bloodstream. Include the roles of key ions and cellular structures.
Mark scheme:
Award 1 mark for each valid point, up to a maximum of 5 marks:
Glucose enters the beta cell through GLUT2 transporters when blood glucose levels rise. (1 mark)
Glucose is metabolised, producing ATP in the mitochondria. (1 mark)
Increased ATP closes ATP-sensitive potassium channels, reducing potassium efflux. (1 mark)
The membrane depolarises, which opens voltage-gated calcium channels, allowing Ca²⁺ ions to enter the cytoplasm. (1 mark)
The rise in Ca²⁺ concentration triggers exocytosis of insulin-containing vesicles. (1 mark)
Accept additional relevant points such as reference to synaptotagmin, SNARE proteins, or the negative feedback role of insulin (maximum 5 marks).

