OCR Specification focus:
‘Explain insulin and glucagon as a negative feedback pair and the liver’s role in maintaining blood glucose.’
Regulation of Blood Glucose Concentration
Maintaining stable blood glucose concentration is vital for energy balance and cellular respiration. Glucose levels fluctuate with diet, metabolism, and activity; homeostatic control ensures they remain within safe limits.
Importance of Blood Glucose Regulation
Glucose provides the primary substrate for aerobic respiration, essential for ATP synthesis. If blood glucose falls too low (hypoglycaemia), cells, particularly neurons, suffer energy deprivation. Conversely, if it rises too high (hyperglycaemia), osmotic imbalance damages cells and long-term vascular health is impaired. Thus, the body tightly regulates glucose concentration, typically around 90 mg per 100 cm³ of blood.
Role of the Liver in Glucose Homeostasis
The liver acts as the main metabolic hub for regulating glucose storage, conversion, and release. It maintains the glucose balance through three primary processes:
Glycogenesis – conversion of glucose to glycogen for storage.
Glycogenolysis – breakdown of glycogen into glucose.
Gluconeogenesis – synthesis of glucose from non-carbohydrate sources such as amino acids and glycerol.
Hepatic Control
The liver receives blood from the hepatic portal vein, rich in nutrients absorbed from the intestine. It monitors and adjusts glucose concentrations by responding to hormonal signals from the pancreas. Hepatocytes (liver cells) possess receptors for these hormones, allowing rapid metabolic adjustments.
The Pancreas and Endocrine Regulation
The pancreas is both an endocrine and exocrine gland. Its islets of Langerhans contain two main types of endocrine cells responsible for glucose regulation:
Alpha (α) cells – secrete glucagon, which increases blood glucose concentration.
Beta (β) cells – secrete insulin, which decreases blood glucose concentration.
These hormones operate as a negative feedback pair, ensuring glucose levels remain stable.
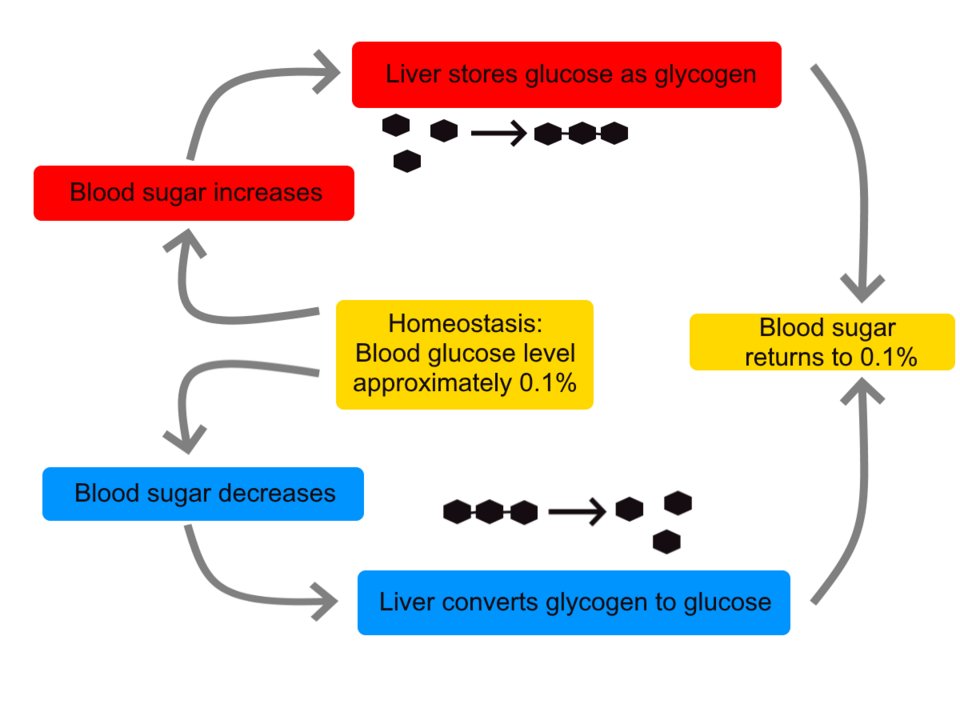
Diagram of blood glucose homeostasis showing insulin promoting glycogenesis and glucagon promoting glycogenolysis to restore levels towards a set point. Arrows link pancreas and liver, emphasising the feedback loop required by the specification. The content maps directly to OCR’s focus on insulin, glucagon and the liver’s role. Source.
Negative Feedback Mechanism
When Blood Glucose Rises (After a Meal)
Elevated glucose levels are detected by β-cells in the pancreas. This triggers insulin secretion, leading to several coordinated effects:
Increased glucose uptake by cells via GLUT4 transporters in muscle and fat membranes.
Stimulation of glycogenesis in the liver and muscles, storing glucose as glycogen.
Enhanced glucose utilisation in respiration.
Inhibition of gluconeogenesis and glycogenolysis, preventing further glucose release.
The result is a return of glucose concentration towards the set point.
Insulin: A peptide hormone secreted by pancreatic β-cells that lowers blood glucose by stimulating glucose uptake and glycogen synthesis.
When Blood Glucose Falls (Between Meals or During Exercise)
Falling glucose levels are detected by α-cells in the pancreas, which release glucagon. Glucagon acts mainly on the liver, promoting:
Glycogenolysis – breakdown of glycogen to glucose.
Gluconeogenesis – synthesis of glucose from amino acids and glycerol.
Reduction of glucose uptake in some tissues to conserve glucose for vital organs.
These actions increase blood glucose, restoring the set point.
Glucagon: A peptide hormone secreted by pancreatic α-cells that increases blood glucose by stimulating glycogen breakdown and gluconeogenesis in the liver.
Beta-Cell Detection and Insulin Secretion Mechanism
Although detailed channel dynamics are covered under a separate subtopic, the essential control principle involves membrane depolarisation in β-cells in response to high glucose concentration. This triggers calcium ion influx and insulin vesicle exocytosis. This mechanism enables rapid and proportional hormone release.
Hormonal Coordination and Target Cells
Insulin’s Mode of Action
Insulin binds to specific receptors on cell membranes in target tissues, particularly liver, muscle, and adipose cells. Binding triggers a signal transduction pathway leading to:
Movement of GLUT4 glucose transporters to the cell surface.
Activation of enzymes promoting glycogen synthesis and glycolysis.
Conversion of excess glucose to lipids for long-term storage.
Glucagon’s Mode of Action
Glucagon acts through a second messenger system involving cyclic AMP (cAMP). When glucagon binds to hepatocyte receptors:
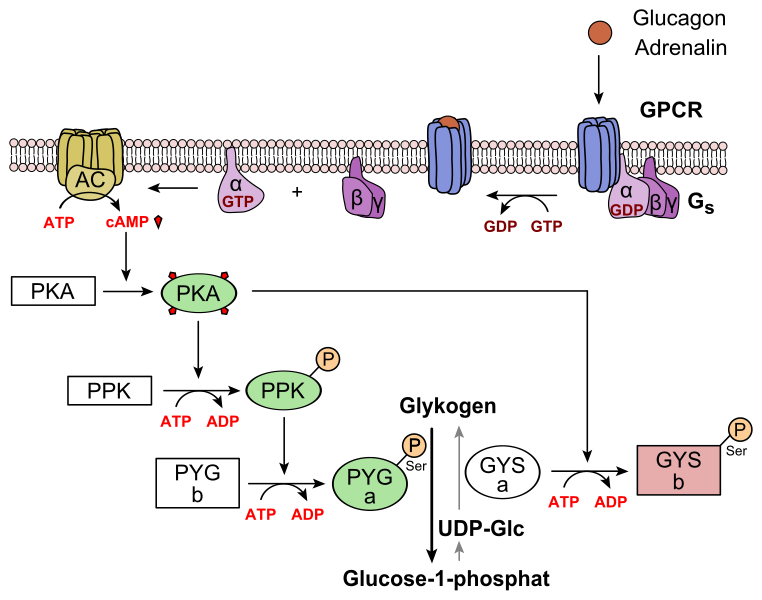
Mechanism diagram of glucagon signalling in hepatocytes: receptor activation → adenylyl cyclase → cAMP/PKA → activation of glycogen phosphorylase and inhibition of glycogen synthase, promoting glycogenolysis. This deepens the OCR-aligned point that glucagon raises blood glucose via the liver. The inclusion of epinephrine as an alternative stimulus is additional context and not required by OCR. Source.
Adenylyl cyclase is activated, converting ATP to cAMP.
cAMP activates protein kinase enzymes.
These enzymes phosphorylate target proteins, promoting glycogen breakdown and inhibiting glycogen synthesis.
Second Messenger: A molecule inside a cell that transmits a signal from a receptor to target molecules within the cytoplasm.
Negative Feedback in Glucose Regulation
The interaction between insulin and glucagon exemplifies negative feedback, where deviation from the normal range triggers a response to reverse the change.
If glucose increases, insulin acts to lower it.
If glucose decreases, glucagon acts to raise it.
When the glucose concentration returns to normal, secretion of the respective hormone is inhibited, stabilising blood glucose around a physiological set point.
Summary of Key Processes
Homeostasis maintains internal stability using feedback mechanisms.
Pancreatic α and β cells detect and respond directly to blood glucose concentration.
Liver metabolism integrates hormonal control through glycogenesis, glycogenolysis, and gluconeogenesis.
Insulin and glucagon act antagonistically, forming a negative feedback pair.
Behavioural and dietary factors, such as food intake and exercise, influence the rate of feedback correction.
Integration with Wider Physiology
Regulation of blood glucose is essential not only for energy metabolism but also for maintaining osmotic balance and supporting other homeostatic systems. The process demonstrates the coordination between endocrine control and target organ response, ensuring that cells receive a constant and appropriate supply of glucose for efficient cellular respiration and survival.
FAQ
Adrenaline complements glucagon’s action during stress or exercise by stimulating glycogen breakdown in the liver and muscles. It binds to receptors on hepatocytes, activating the cAMP second messenger system, similar to glucagon, which triggers glycogenolysis and increases blood glucose availability.
This ensures rapid glucose release to supply energy to muscles during the “fight or flight” response, even when glucagon alone may not be sufficient.
Neurons cannot metabolise fatty acids efficiently because they lack the necessary enzymes for β-oxidation and cannot access lipids easily through the blood–brain barrier.
Therefore, glucose provides a continuous and immediate energy supply for ATP production through respiration, ensuring proper function of nerve impulses and synaptic transmission. If glucose falls too low, brain activity becomes impaired, leading to confusion, seizures, or unconsciousness.
Although both are peptide hormones, hepatocytes express different membrane receptors specific to each hormone.
Insulin binds to tyrosine kinase receptors, activating enzyme cascades promoting glycogen synthesis and glucose uptake.
Glucagon binds to G protein-coupled receptors, activating adenylyl cyclase and raising cAMP levels, leading to glycogen breakdown.
Thus, distinct receptor mechanisms allow opposite metabolic responses within the same cell type.
Sensitivity depends on the number and responsiveness of insulin receptors on target cells.
Factors that decrease sensitivity include:
Obesity and high-fat diets, which reduce receptor expression.
Prolonged high insulin levels, causing receptor desensitisation.
Inflammatory signals and lipid accumulation interfering with signalling pathways.
Improved sensitivity can result from regular exercise and healthy diet, which increase receptor numbers and glucose transporter availability.
Glycogen is a branched polysaccharide made of glucose monomers linked by α-1,4 and α-1,6 glycosidic bonds.
Its structure offers:
Compact storage due to coiling and branching.
Rapid mobilisation, as many enzymes can act simultaneously on branch ends during glycogenolysis.
Osmotic stability, since it does not dissolve or affect water potential significantly.
This makes glycogen ideal for maintaining blood glucose balance without disrupting cell osmotic conditions.
Practice Questions
Question 1 (2 marks)
Explain how the hormones insulin and glucagon work as a negative feedback pair to maintain blood glucose concentration.
Mark Scheme:
1 mark for stating that insulin decreases and glucagon increases blood glucose concentration.
1 mark for describing that each hormone’s secretion is inhibited once blood glucose returns to normal, demonstrating negative feedback control.
Question 2 (5 marks)
Describe the role of the liver in the regulation of blood glucose concentration and explain how hormonal control ensures glucose levels remain within a normal range.
Mark Scheme:
1 mark for identifying that the liver stores and releases glucose to maintain blood glucose concentration.
1 mark for describing glycogenesis (conversion of glucose to glycogen) under the influence of insulin.
1 mark for describing glycogenolysis and/or gluconeogenesis under the influence of glucagon.
1 mark for stating that insulin and glucagon act antagonistically in a negative feedback mechanism.
1 mark for linking hormonal action to restoration of blood glucose to the normal range (set point).

