OCR Specification focus:
‘Write electron configurations for Period 4 d-block atoms and ions using sub-shell notation.’
Period 4 d-block elements display characteristic electron configurations that underpin their chemistry. Understanding sub-shell notation allows prediction of atomic and ionic structures essential for later transition-metal topics.
Electron Configurations of Period 4 d-Block Elements
Period 4 contains the ten d-block elements from scandium (Sc) to zinc (Zn). In the OCR A-Level course, students must be able to write their electron configurations accurately in sub-shell notation, including the configurations of their common ions. These configurations provide the foundation for explaining their variable oxidation states, coloured ions, and complex formation encountered later in the transition-metal topic.
Before examining individual atoms and ions, it is essential to recognise how electrons fill the 4s and 3d sub-shells.
Sub-Shell Filling Order
Electrons occupy orbitals in order of increasing energy. Period 4 d-block elements follow a characteristic filling pattern: the 4s orbital is filled before the 3d orbitals when writing configurations for neutral atoms. This ordering is crucial for correct notation.
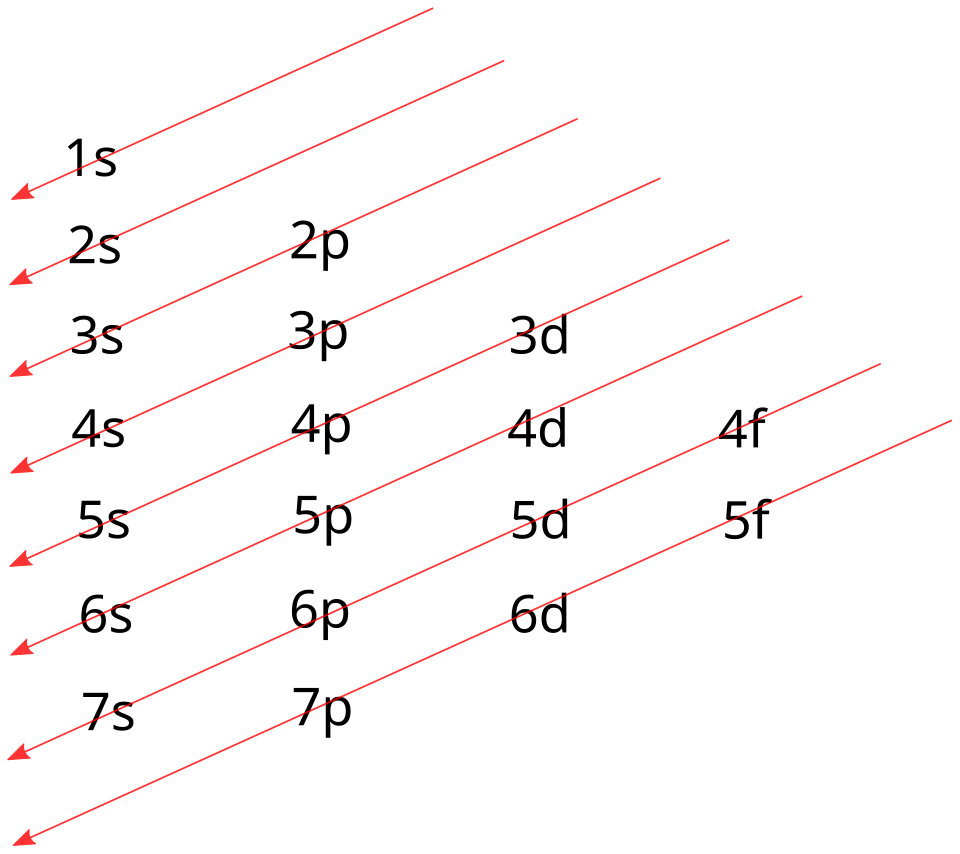
This diagram shows the orbital-filling order used to write electron configurations. Following the diagonal arrows gives the standard sequence, including 4s filling before 3d in Period 4. Source
Electron configuration: The arrangement of electrons in atomic orbitals written using principal quantum numbers and sub-shell labels.
The filling pattern continues through the series as electrons populate the five 3d orbitals. Each 3d orbital can hold a pair of electrons, giving a total of ten electrons in the 3d sub-shell.
Key features of filling behaviour
The 4s sub-shell is always written before 3d in configuration notation for neutral atoms because it is filled first.
The 3d sub-shell fills progressively from Sc (3d¹) to Zn (3d¹⁰).
Electrons obey the Pauli Exclusion Principle and Hund’s Rule, ensuring correct arrangement within orbitals, though detailed orbital diagrams are not required by the specification.
A further important point emerges when writing configurations for ions, especially those of transition metals.
Electron Removal in Ion Formation
When Period 4 d-block elements form ions, the energy ordering shifts so that electrons are removed from the 4s sub-shell before the 3d sub-shell. This point is frequently tested and must be emphasised.
Ion: A charged species formed by the loss or gain of electrons.
Even though the 4s fills first, it is always the first to lose electrons during ionisation. This reversal highlights that sub-shell energy ordering differs between isolated atoms and their ions.
A short sentence is placed here to ensure spacing before the next definition.
Transition element (OCR context): A d-block element that forms ions with an incomplete d sub-shell.
Rules for writing ion configurations
Write the neutral atom configuration first.
Remove electrons from 4s before 3d.
Ensure sub-shell notation remains in the order: 1s, 2s, 2p, 3s, 3p, 4s, 3d, 4p (even though 4s electrons are lost first).
This distinction becomes especially significant for elements such as titanium, vanadium, chromium, manganese, and copper, which form multiple oxidation states.
Period 4 d-Block Elements: Atomic Electron Configurations
The neutral atoms follow a broadly predictable pattern. Students must know how to derive these using the Aufbau principle and apply sub-shell notation correctly.
General trends across the series
Sc (Z=21): 4s² 3d¹
Ti (Z=22): 4s² 3d²
V (Z=23): 4s² 3d³
Cr (Z=24): Exception – 4s¹ 3d⁵ (stabilised half-filled d sub-shell)
Mn (Z=25): 4s² 3d⁵
Fe (Z=26): 4s² 3d⁶
Co (Z=27): 4s² 3d⁷
Ni (Z=28): 4s² 3d⁸
Cu (Z=29): Exception – 4s¹ 3d¹⁰ (stabilised full d sub-shell)
Zn (Z=30): 4s² 3d¹⁰
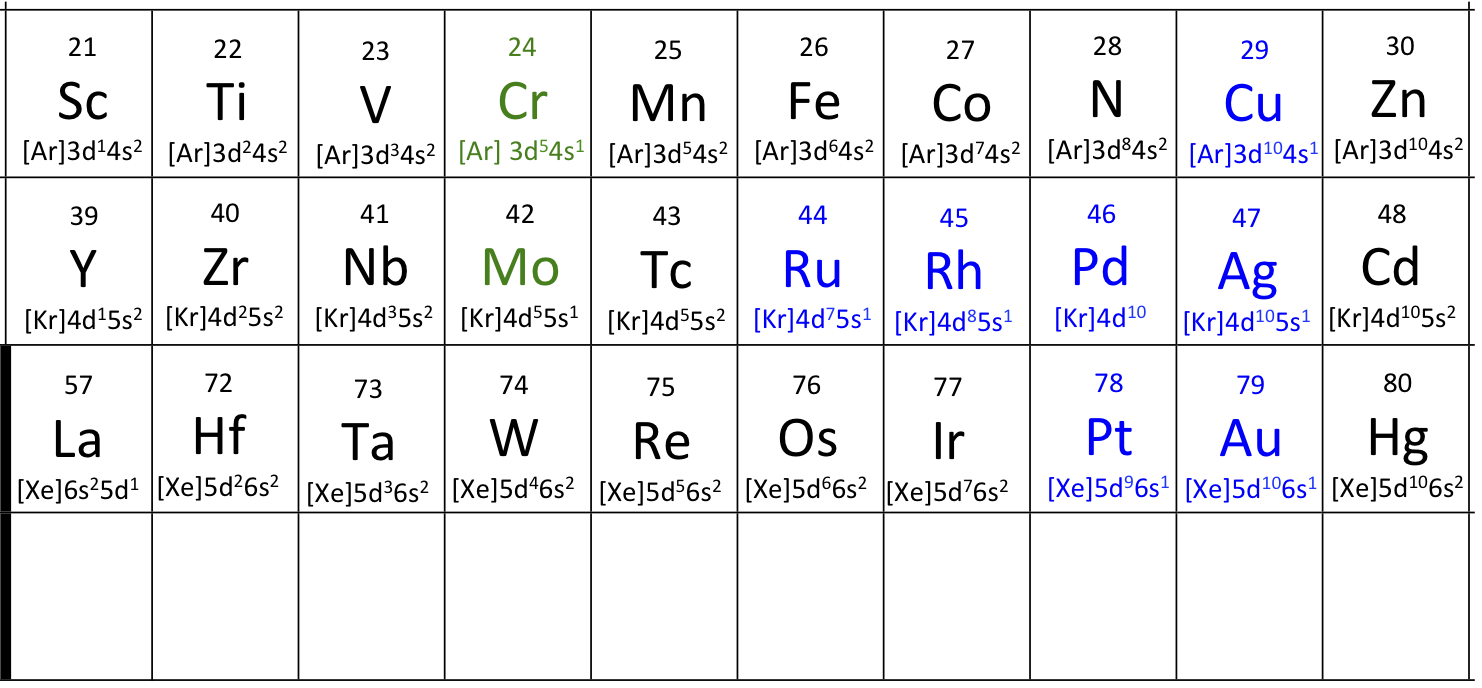
This diagram summarises the valence electron configurations of the Period 4 transition metals using [Ar] 3dⁿ 4sᵐ notation. It highlights the chromium and copper exceptions where a 4s electron is promoted for added d-subshell stability. Source
Notes on the exceptions
Chromium and copper depart from the expected 4s² 3d⁴ and 4s² 3d⁹ patterns.
Half-filled (d⁵) and fully filled (d¹⁰) sub-shells offer added stability due to symmetrical electron distribution and reduced electron repulsion.
These exceptions are required knowledge for accurate atomic and ionic configuration writing.
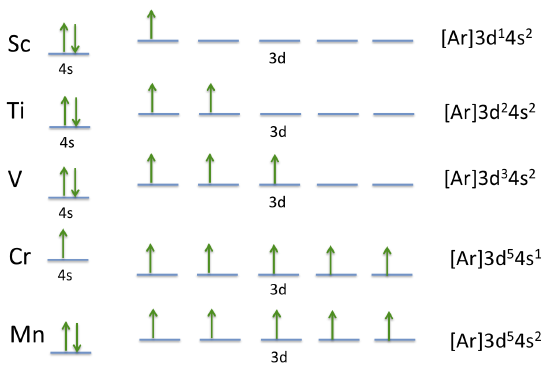
These orbital diagrams illustrate how electrons are distributed between 4s and 3d orbitals for early Period 4 transition metals. Arrow direction shows electron spin, which is additional detail beyond the syllabus minimum, while the written configurations emphasise the chromium 3d⁵ 4s¹ exception. Source
Period 4 d-Block Ions: Electron Configurations
Students must also write configurations for common ions such as Sc³⁺, Ti³⁺/Ti²⁺, V³⁺/V²⁺, Cr³⁺, Mn²⁺, Fe²⁺/Fe³⁺, Co²⁺, Ni²⁺, Cu⁺/Cu²⁺, and Zn²⁺.
Although the exact list is not specified, these ions commonly appear across OCR questions.
General principles
Remove the 4s electrons first (up to two electrons).
Then remove electrons from the 3d sub-shell as needed to match the ionic charge.
Examples of typical patterns (non-worked, structural only)
Ti: Ti → [Ar] 4s² 3d² → Ti³⁺: [Ar] 3d¹
Mn: Mn → [Ar] 4s² 3d⁵ → Mn²⁺: [Ar] 3d⁵
Fe: Fe → [Ar] 4s² 3d⁶ → Fe³⁺: [Ar] 3d⁵
Cu: Cu → [Ar] 4s¹ 3d¹⁰ → Cu²⁺: [Ar] 3d⁹
Zn: Zn → [Ar] 4s² 3d¹⁰ → Zn²⁺: [Ar] 3d¹⁰
These structural patterns help students recognise which ions maintain partially filled d sub-shells and therefore meet the OCR definition of a transition element.
Why Electron Configurations Matter in Later Topics
Understanding electron configurations for Period 4 d-block elements is essential preparation for the remainder of Topic 11. Ion configurations underpin:
Variable oxidation states (11.1.3)
Coloured ions arising from d–d transitions (11.1.3)
Formation of complex ions with ligands (11.1.4)
Ligand substitution and stability in biological systems (11.1.6)
Although these ideas appear in later sections, they rely directly on the ability to write configurations in correct sub-shell notation as required in this subsubtopic.
FAQ
The order reflects how electrons are added to an atom, not how they are removed.
For isolated atoms, the 4s sub-shell is slightly lower in energy than 3d, so it fills first.
When an atom forms ions, electron–electron repulsion and shielding change the energy order, making 4s higher in energy than 3d and therefore lost first.
No, only chromium and copper show stable exceptions in their ground-state configurations.
These occur because half-filled (3d5) and fully filled (3d10) d sub-shells are especially stable.
Other elements do not gain enough stability from electron rearrangement to outweigh the energy cost.
Using a noble gas core simplifies long configurations while keeping essential information clear.
It allows focus on the valence electrons in the 4s and 3d sub-shells, which determine chemical behaviour.
For Period 4 d-block elements, the core is always [Ar].
Look only at the number of electrons in the 3d sub-shell.
If the 3d sub-shell contains between 1 and 9 electrons, it is incomplete.
If it contains 0 or 10 electrons, it is complete and the ion is not a transition element by the OCR definition.
Zinc’s position places it in the d-block, but its chemistry differs from typical transition elements.
Both Zn atoms and Zn2+ ions have a full 3d10 sub-shell.
Because none of its ions have an incomplete d sub-shell, zinc does not meet the OCR definition of a transition element.
Practice Questions
Write the full sub-shell electron configuration of the Fe3+ ion.
(2 marks)
1 mark for correctly removing electrons from the 4s sub-shell first.
1 mark for the correct final configuration:
1s2 2s2 2p6 3s2 3p6 3d5
(a) Write the full sub-shell electron configuration of a neutral chromium atom.
(b) Write the full sub-shell electron configuration of the Cr3+ ion.
(c) Explain why the electron configuration of chromium is considered an exception compared with neighbouring d-block elements.
(5 marks)
(a) Neutral chromium atom (2 marks)
1 mark for recognising chromium as an exception.
1 mark for the correct configuration:
1s2 2s2 2p6 3s2 3p6 3d5 4s1
(b) Cr3+ ion (2 marks)
1 mark for removing electrons from the 4s sub-shell before the 3d sub-shell.
1 mark for the correct configuration:
1s2 2s2 2p6 3s2 3p6 3d3
(c) Explanation (1 mark)
1 mark for stating that a half-filled 3d sub-shell (3d5) is more stable due to reduced electron repulsion or symmetrical electron arrangement.

