OCR Specification focus:
‘Define transition elements (Ti–Cu) as d-block elements with ions having incomplete d sub-shells.’
Transition elements occupy a central position in inorganic chemistry, linking atomic structure to chemical behaviour and explaining many distinctive properties observed in transition metal compounds.
What Is Meant by a Transition Element?
The term transition element has a precise meaning in A-Level Chemistry and does not simply refer to position in the Periodic Table. Although several elements lie in the d-block, only some qualify as true transition elements under the OCR definition.
Transition element: A d-block element that forms at least one ion with an incomplete d sub-shell.
This definition emphasises ionic electronic structure rather than the neutral atom. An element must be capable of forming an ion with partially filled d-orbitals to be classified as a transition element.
The d-Block and Period 4 Elements
The d-block consists of elements where electrons are added to a d sub-shell. In Period 4, these elements extend from scandium (Sc) to zinc (Zn).
d-block element: An element whose atoms or common ions involve the filling of d orbitals.
For OCR A-Level Chemistry, the relevant transition elements are titanium (Ti) to copper (Cu). Although scandium and zinc sit in the d-block, they are excluded from the transition element definition due to their ionic electron configurations.
On the periodic table, the Period 4 d-block runs from Sc to Zn, but OCR defines the transition elements as Ti–Cu because their ions can have incomplete d sub-shells.
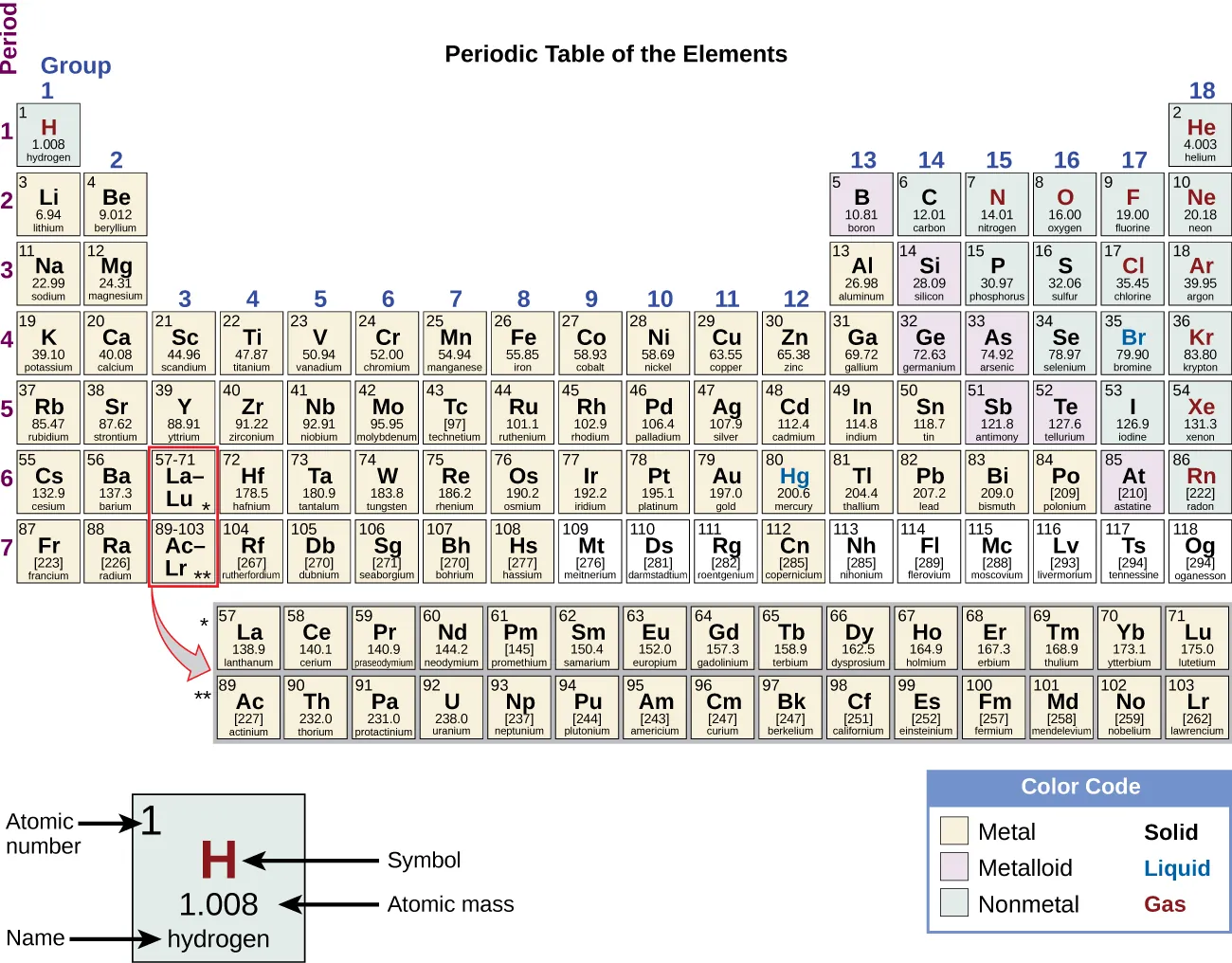
This periodic table highlights the central region where the d-block sits, including the first transition series. For OCR, focus on Ti–Cu within Period 4; the diagram also shows other d-block and inner transition regions that go beyond this subsubtopic. Source
Incomplete d Sub-Shells Explained
A d sub-shell can hold a maximum of ten electrons. When it contains between one and nine electrons, it is described as incomplete. This incomplete nature is crucial because it enables the characteristic chemistry of transition elements.
Incomplete d sub-shell: A d orbital set containing fewer than ten electrons.
Ions with incomplete d sub-shells allow:
Variable oxidation states
Formation of coloured compounds
Ability to act as catalysts
These properties arise from the involvement of d electrons in bonding and electron transitions.
Why Ions Matter More Than Atoms
The OCR definition focuses on ions, not neutral atoms. Some elements have incomplete d sub-shells as atoms but form ions with full or empty d sub-shells, disqualifying them as transition elements.
For example:
Scandium (Sc) forms Sc³⁺ with a 3d⁰ configuration
Zinc (Zn) forms Zn²⁺ with a 3d¹⁰ configuration
Both ions have complete or empty d sub-shells, so neither element is classed as a transition element, despite their position in the d-block.
Accepted Transition Elements: Titanium to Copper
The elements from titanium to copper form ions that retain incomplete d sub-shells. This is why OCR explicitly defines the transition elements as Ti–Cu.
Examples include:
Ti²⁺ and Ti³⁺ with partially filled 3d orbitals
Fe²⁺ (3d⁶) and Fe³⁺ (3d⁵)
Cu²⁺ (3d⁹)
In each case, the ion formed has an incomplete d sub-shell, satisfying the definition.
Distinguishing Transition Elements from Other Metals
Transition elements differ from s-block metals and non-transition d-block elements in several fundamental ways due to their electronic structures.
Key distinctions include:
Presence of d electrons in ions
Greater variety of stable ions
Strong tendency to form complex ions
These features all stem from the incomplete d sub-shells specified in the OCR definition.
Transition elements are part of the d-block, meaning their distinguishing electrons (or vacancies) are in d orbitals rather than s or p orbitals.
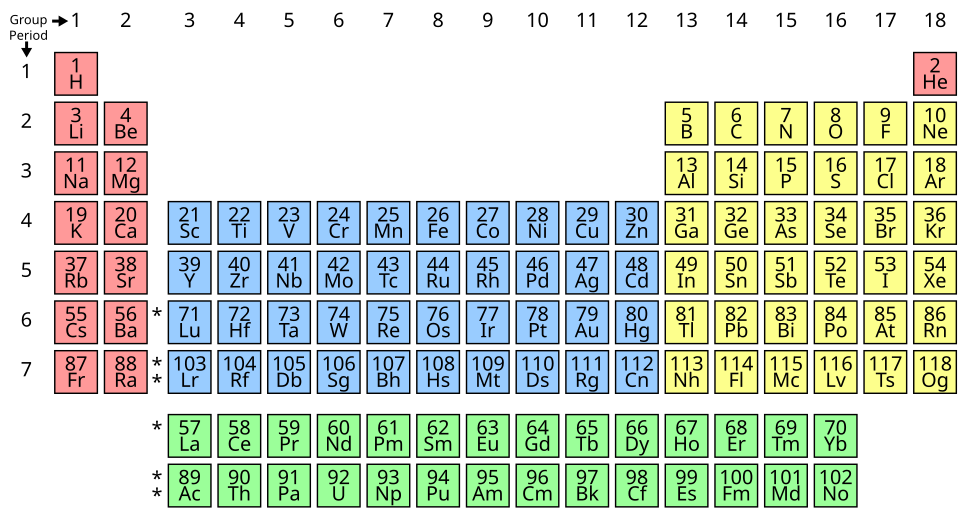
This diagram divides the periodic table into s-, p-, d-, and f-blocks based on the subshell being filled. For this subsubtopic, use it to locate the d-block (centre) and then narrow attention to Period 4 within that block; the f-block shown is extra context not required here. Source
Importance of the OCR Definition
Understanding the precise definition prevents common misconceptions, particularly the assumption that all d-block elements are transition elements. Examiners expect students to apply the ionic definition accurately, especially when justifying classifications.
When asked to identify or define transition elements:
Always refer to ions, not atoms
State the requirement for an incomplete d sub-shell
Restrict examples to Ti–Cu
This approach ensures alignment with the specification and avoids incorrect inclusion of scandium or zinc.
Summary of Key Criteria
To qualify as a transition element under OCR A-Level Chemistry, an element must:
Be located in the d-block
Form at least one stable ion
Have an incomplete d sub-shell in that ion
These criteria underpin all subsequent study of transition element chemistry, including colour, catalysis, complex formation, and redox behaviour.
FAQ
The chemistry of transition elements is dominated by their ions in compounds and solutions, not by isolated atoms.
Ions determine properties such as colour, magnetism, and catalytic behaviour, all of which depend on d-electron arrangements. Focusing on ions ensures the definition reflects chemical behaviour rather than atomic position alone.
Yes, provided it can form at least one stable ion with an incomplete d sub-shell.
Some elements have a most common ion with a full or empty d sub-shell but still meet the definition if an alternative ion with an incomplete d sub-shell exists.
Both elements form ions with either empty or completely filled d sub-shells.
As a result, they cannot exhibit the characteristic chemistry associated with partially filled d orbitals, which is why they fail the OCR definition despite their d-block position.
An element must be able to form ions with between one and nine d electrons.
This range allows d electrons to participate in bonding and electron transitions, which is essential for the defining chemical behaviour of transition elements.
This restriction ensures all included elements consistently meet the ionic definition.
Titanium to copper reliably form ions with incomplete d sub-shells, whereas scandium and zinc do not, making the classification clear and unambiguous for assessment purposes.
Practice Questions
Define a transition element according to the OCR A-Level Chemistry specification and name one Period 4 element that is not classed as a transition element.
(2 marks)
1 mark for stating that a transition element is a d-block element that forms at least one ion with an incomplete d sub-shell
1 mark for correctly naming a Period 4 element that is not a transition element, such as scandium or zinc
Explain why zinc is not classified as a transition element, even though it is located in the d-block of the Periodic Table. In your answer, refer to electron configurations and the definition of a transition element.
(5 marks)
1 mark for stating that zinc is a d-block element
1 mark for stating that zinc commonly forms the Zn2+ ion
1 mark for identifying the electron configuration of Zn2+ as having a full d sub-shell (3d10)
1 mark for explaining that a full d sub-shell is not incomplete
1 mark for linking this to the definition of a transition element, concluding that zinc does not meet the requirement of forming an ion with an incomplete d sub-shell

