OCR Specification focus:
‘Describe cis–trans and optical isomerism in complexes; explain cis-platin’s action binding DNA to prevent cell division.’
Transition-metal complexes show important three-dimensional arrangements of ligands. Understanding their cis–trans and optical isomerism is essential, particularly for appreciating the medicinal significance of cis-platin in cancer treatment.
Stereoisomerism in Transition-Metal Complexes
Stereoisomerism arises when species share the same molecular formula and bonding connectivity but differ in the spatial arrangement of ligands around the central metal ion. In transition-metal chemistry, stereoisomerism becomes especially significant due to the presence of complex ion geometries, such as octahedral, square planar, and tetrahedral structures. For OCR A-Level Chemistry, the specification highlights two key forms of stereoisomerism: cis–trans isomerism and optical isomerism, both of which commonly occur in coordination complexes.
Cis–Trans Isomerism
Cis–trans isomerism occurs when identical ligands in a complex occupy either adjacent (cis) or opposite (trans) positions. This commonly appears in octahedral complexes with formula [ML₄A₂] and square planar complexes with formula [MA₂B₂].
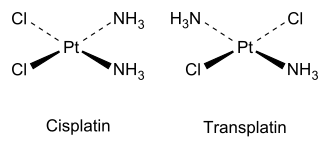
This diagram compares the cis and trans geometric isomers of a square planar platinum(II) complex, showing how ligand positions differ. In cisplatin, identical ligands are adjacent; in transplatin, they are opposite. Source
Cis–trans isomerism: Isomerism in which two identical ligands are arranged either next to each other (cis) or opposite each other (trans) around a central metal ion.
In octahedral complexes, cis isomers place the two identical ligands at a bond angle of 90°, whereas trans isomers place them 180° apart. This difference significantly alters physical properties, including colour, solubility, reactivity, and, for biologically active complexes, physiological effects. Square planar complexes also readily exhibit this isomerism, where the ligand arrangement in one plane around the metal leads to clear geometric alternatives.
Key cases where cis–trans isomerism is important include:
Complexes of Pt(II), particularly those with the general structure [Pt(NH₃)₂Cl₂].
Octahedral complexes such as [Co(NH₃)₄Cl₂]⁺.
Complexes containing monodentate ligands that differ in donor atoms.
Optical Isomerism
Optical isomerism occurs when complexes exist as non-superimposable mirror images, known as enantiomers, which rotate plane-polarised light in opposite directions. This arises mainly in octahedral complexes containing bidentate ligands, such as ethane-1,2-diamine (en) or oxalate (C₂O₄²⁻).
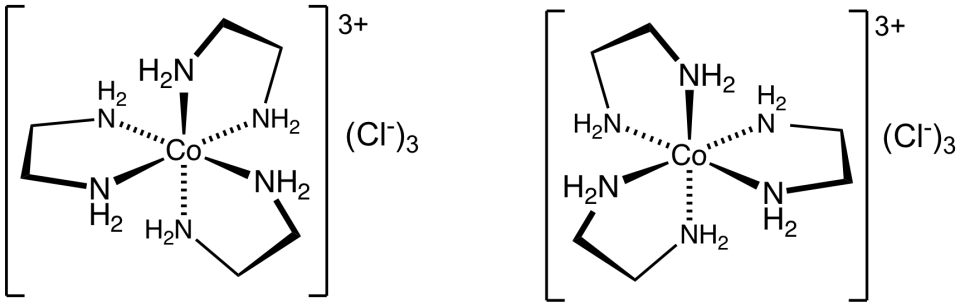
The image shows two enantiomers of an octahedral tris(ethylenediamine)cobalt(III) complex. The ligands wrap around the metal ion in opposite handed arrangements, producing non-superimposable mirror images. Source
Optical isomerism: A form of stereoisomerism in which molecules or complexes exist as non-superimposable mirror images that rotate plane-polarised light.
These complexes possess a chiral centre, often created when the positions of the bidentate ligands create a helical twist around the central metal ion. Chirality is particularly common in [M(en)₃]ⁿ⁺ or [M(C₂O₄)₃]ⁿ⁻ complexes, where the three bidentate ligands wrap around the metal in two distinct mirror-image ways. Optical isomers generally share identical physical properties except for how they interact with plane-polarised light and chiral biological systems, making their study important in medicinal chemistry.
A sentence to separate definition blocks: Optical activity in transition-metal complexes becomes more significant when the arrangement of ligands restricts free rotation, locking the structure into a chiral form.
Cis-Platin and Its Medicinal Importance
The OCR specification requires understanding the action of cis-platin, a crucial anti-cancer drug. Cis-platin, cis-[Pt(NH₃)₂Cl₂], is a square planar complex of platinum(II) whose biological activity depends entirely on its cis geometry. The trans isomer, trans-platin, does not show comparable anti-cancer activity, which illustrates the profound effect stereochemistry has on biological behaviour.
Structure and Stereochemistry of Cis-Platin
In cis-platin, the two chloride ligands sit adjacent to each other, allowing sequential ligand substitution reactions once the drug enters the body. Because chloride concentration in cells is lower than in the bloodstream, chloride ligands on cis-platin are readily replaced by water molecules. This activation step produces a positively charged aqua complex, which is far more reactive toward nucleophilic sites in DNA.
Key features of the cis-platin geometry include:
Square planar arrangement around Pt(II).
Cis positioning of chloride ligands enabling bidentate-type binding to biological targets.
Two NH₃ ligands acting as spectator ligands that stabilise the complex.
Binding to DNA and Prevention of Cell Division
Cis-platin exerts its medicinal effect by binding to DNA, specifically to adjacent guanine bases.
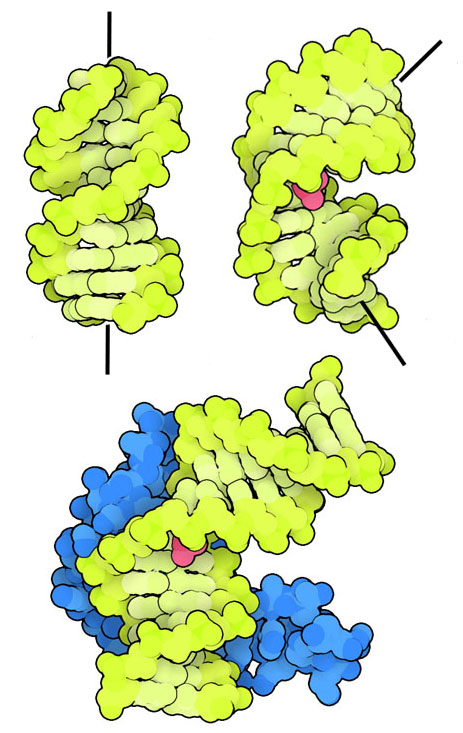
This illustration shows how cisplatin binding causes the DNA double helix to bend, disrupting normal replication. The additional protein shown recognises the bent DNA and goes beyond OCR requirements but reinforces the biological significance of the distortion. Source
The geometry of the cis isomer enables the platinum centre to coordinate to two neighbouring N atoms on guanine through ligand substitution, creating intrastrand cross-links.
The process can be summarised as follows:
Chloride ligands are replaced by water molecules inside the cell.
Aqua ligands are displaced by DNA bases with lone-pair nitrogen donors.
Pt forms two coordinate bonds to adjacent guanine residues.
DNA becomes kinked and unable to replicate or undergo normal transcription.
These structural distortions halt DNA replication, ultimately triggering apoptosis in rapidly dividing cancer cells. Because of this mechanism, cis-platin is highly effective against testicular, ovarian, bladder, and lung cancers.
A normal sentence between lists: The success of cis-platin depends not only on its reactivity but also on its precise stereochemical arrangement, highlighting the link between coordination chemistry and therapeutic function.
Differences Between Cis-Platin and Trans-Platin
The contrasting behaviours of cis-platin and trans-platin demonstrate the fundamental importance of stereochemistry in drug design.
Key distinctions:
Cis-platin can coordinate with two adjacent DNA bases, forming stable cross-links.
Trans-platin positions its chloride ligands opposite each other, preventing effective simultaneous binding to neighbouring guanine bases.
Trans-platin therefore fails to disrupt DNA replication in the same way and lacks significant anti-cancer activity.
Stereochemical constraints thus dictate biological and medicinal outcomes, reinforcing the importance of accurate three-dimensional understanding in transition-metal chemistry.
FAQ
Cis–trans isomerism requires ligands to occupy positions that are clearly adjacent or opposite. In tetrahedral complexes, all bond angles are approximately 109.5°, so no two positions are geometrically opposite.
As a result, rearranging ligands in a tetrahedral complex does not create distinct cis or trans forms. Any apparent rearrangement produces the same spatial arrangement, meaning tetrahedral complexes cannot show cis–trans isomerism.
Bidentate ligands bind to a metal ion through two donor atoms, forming chelate rings. When several bidentate ligands coordinate, they can wrap around the metal in a twisted arrangement.
This wrapping can produce:
Left-handed and right-handed forms
Non-superimposable mirror images
Monodentate ligands usually allow too much symmetry or rotation, preventing chirality from arising.
The behaviour of cis-platin depends on chloride ion concentration. Blood plasma contains a high concentration of Cl⁻ ions, stabilising the complex.
Inside cells:
Chloride concentration is much lower
Chloride ligands are replaced by water molecules
The complex becomes more reactive
This change allows cis-platin to bind to DNA only after entering cells, reducing damage to healthy tissues.
Cis-platin disrupts DNA replication by forming cross-links between DNA bases. Rapidly dividing cells rely heavily on frequent DNA replication and transcription.
Cancer cells divide more often than normal cells, so they are:
More likely to encounter cis-platin–DNA cross-links
Less able to repair the resulting DNA damage
This selectivity explains why cis-platin is particularly effective as a chemotherapy agent.
Biological molecules such as enzymes and DNA are chiral, meaning they can distinguish between optical isomers.
As a result:
One enantiomer may bind effectively to a biological target
The other may be less active or inactive
This is why the spatial arrangement of ligands is crucial when designing metal-based drugs, even if the chemical formula is unchanged.
Practice Questions
Define cis–trans isomerism in transition-metal complexes and state one structural condition required for this type of isomerism to occur.
(2 marks)
Award marks as follows:
1 mark for defining cis–trans isomerism as arising from different spatial arrangements of identical ligands (adjacent or opposite) around a metal ion.
1 mark for stating a correct structural condition, such as:
square planar or octahedral geometry
presence of two identical ligands in different positions
Cis-platin, cis-[Pt(NH3)2Cl2], is an important anti-cancer drug, whereas its trans isomer has little biological activity.
Explain why cis-platin is able to prevent cell division, referring to its stereochemistry and its interaction with DNA.
(5 marks)
Award marks as follows:
1 mark for recognising that cis-platin is square planar and exists as a cis isomer.
1 mark for stating that the two chloride ligands are adjacent in the cis isomer.
1 mark for describing ligand substitution, where chloride ligands are replaced by water molecules in the cell.
1 mark for explaining that cis-platin binds to DNA by forming coordinate bonds to adjacent guanine bases.
1 mark for linking DNA binding to distortion of the DNA structure, preventing replication or cell division.
Notes:
Answers must refer specifically to the cis arrangement; generic descriptions of platinum drugs without stereochemistry gain a maximum of 2 marks.
Reference to trans-platin being ineffective due to ligand positions may be credited within the explanation but is not required for full marks.

