OCR Specification focus:
‘Explain ligands and coordinate bonding; define complex ion and coordination number; recognise octahedral, tetrahedral, square planar.’
Transition metal chemistry is dominated by complex formation, where metal ions bond to ligands, creating distinctive structures, shapes, and chemical behaviour essential to coordination chemistry.
Ligands and Coordinate Bonding
A ligand is an ion or molecule that donates a pair of electrons to a central metal ion or atom to form a coordinate bond. Ligands must contain at least one lone pair of electrons that can be donated.
Ligand: An ion or molecule that donates a lone pair of electrons to a central metal ion, forming a coordinate (dative covalent) bond.
Coordinate bonding is a specific type of covalent bonding where both electrons in the shared pair originate from the same species, namely the ligand. This contrasts with ordinary covalent bonds, where each atom contributes one electron.
In transition metal complexes:
The metal ion acts as a Lewis acid, accepting electron pairs.
The ligand acts as a Lewis base, donating electron pairs.
The resulting bond is called a coordinate (dative covalent) bond.
Ligands act as Lewis bases, donating a lone pair of electrons to a central metal ion to form a coordinate (dative) bond.
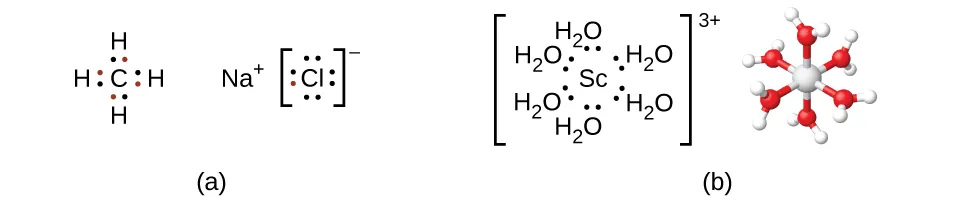
This diagram illustrates coordinate (dative covalent) bonding, where lone pairs from ligands are donated to a central metal ion. Six water ligands donate electron pairs to Sc³⁺, producing an octahedral complex; the covalent and ionic examples provide contextual contrast beyond the transition-metal focus. Source
Common ligands encountered at OCR A-Level include:
H₂O (aqua) – neutral ligand
NH₃ (ammine) – neutral ligand
Cl⁻ (chloro) – negatively charged ligand
CN⁻ (cyano) – negatively charged ligand
Ligands may form one or more coordinate bonds with a metal ion depending on the number of lone pairs available.
Complex Ions
When ligands bond to a central metal ion, a complex ion is formed. The overall charge of the complex depends on the charge of the metal ion and the charges of the ligands attached.
Complex ion: A charged species formed by a central metal ion bonded to one or more ligands via coordinate bonds.
Complex ions are written using square brackets, with the overall charge shown outside the brackets. The name of the complex reflects both the metal ion and the ligands present, though naming conventions are covered elsewhere.
The formation of complex ions explains many characteristic properties of transition metals, including:
High solubility of some metal salts
Formation of vividly coloured solutions
Changes in colour during ligand substitution reactions
Coordination Number
The coordination number describes how many coordinate bonds form between the central metal ion and surrounding ligands.
Coordination number: The number of coordinate bonds formed between a central metal ion and its ligands.
Each coordinate bond corresponds to one donated lone pair from a ligand. The coordination number depends on:
The size of the metal ion
The size and charge of the ligands
Electronic factors within the metal ion
Common coordination numbers for transition metals are 4 and 6, which lead directly to characteristic three-dimensional shapes.
Shapes of Complex Ions
The spatial arrangement of ligands around a central metal ion gives complex ions distinct geometrical shapes. At OCR A-Level, students must recognise three key geometries.
Octahedral Complexes
An octahedral complex has a coordination number of 6, with six ligands arranged symmetrically around the metal ion.
Key features include:
Bond angles of 90°
Typically formed with small ligands such as H₂O or NH₃
Very common among transition metals
Octahedral geometry minimises repulsion between ligands while allowing efficient overlap between ligand lone pairs and metal orbitals.
Tetrahedral Complexes
A tetrahedral complex has a coordination number of 4, with ligands positioned at the corners of a tetrahedron.
Important characteristics:
Bond angles of approximately 109.5°
Often formed with larger ligands such as Cl⁻
Less common than octahedral complexes for transition metals
For coordination number 4, complexes are commonly tetrahedral or square planar depending on the metal ion and ligand set.
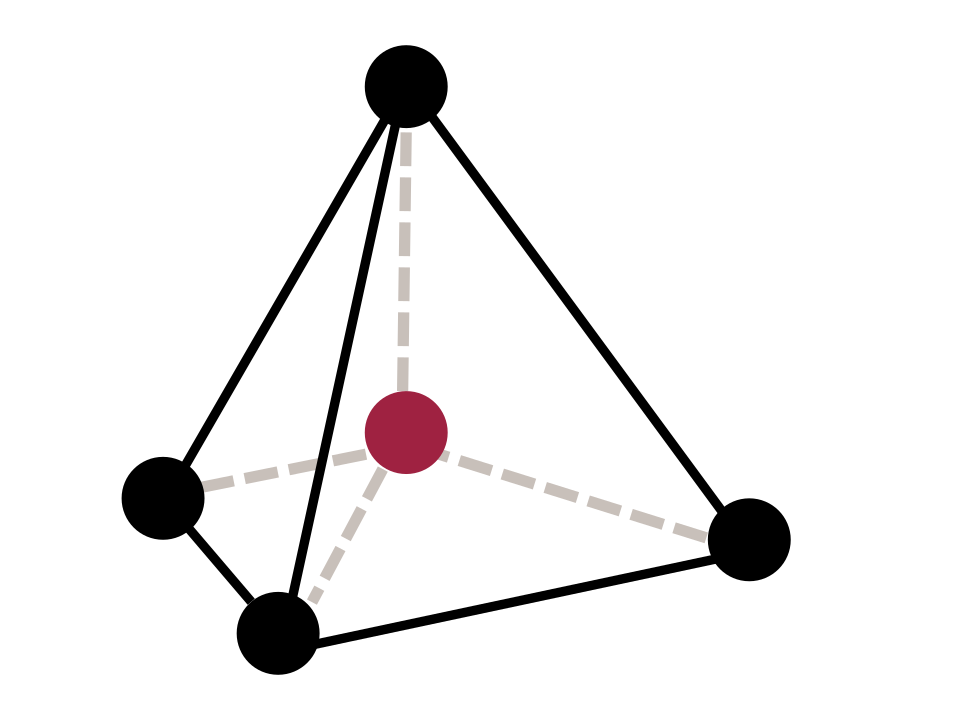
This diagram shows a tetrahedral arrangement in which four ligands surround a central atom with bond angles close to 109.5°. Tetrahedral geometry is one possible structure for coordination number 4 transition-metal complexes. Source
Tetrahedral complexes are typically associated with lower charge density on the metal ion, allowing bulkier ligands to arrange with reduced repulsion.
Square Planar Complexes
A square planar complex also has a coordination number of 4, but ligands lie in a single plane around the metal ion.
Key identifying features:
Bond angles of 90°
Ligands arranged in a flat square shape
Common for certain metal ions, particularly those with specific electronic configurations
In square planar complexes, four ligands lie in one plane with approximately 90° angles around the metal centre.
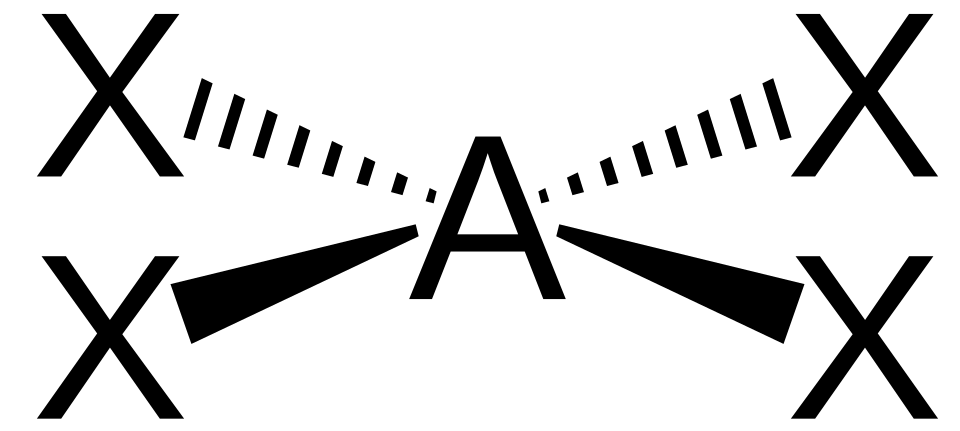
This diagram illustrates square planar geometry, where four ligands are arranged in one plane around a central metal ion. This is a recognised coordination number 4 structure in transition-metal chemistry. Source
Square planar geometry arises due to electronic factors within the metal ion that favour planar arrangements over tetrahedral ones.
Linking Structure to Chemical Behaviour
The coordination number and shape of a complex strongly influence its chemical properties. Different geometries affect:
How complexes interact with other molecules
The ease of ligand substitution
The stability of the complex ion
Understanding ligands, coordinate bonding, and complex geometry provides a foundation for later topics such as stereoisomerism, ligand exchange, and precipitation reactions, all of which rely on these structural principles.
FAQ
Transition metal ions have vacant or partially vacant d orbitals that can accept lone pairs from ligands.
They also have a higher charge density due to their greater nuclear charge and relatively small ionic radius, which strengthens attraction to ligands.
In contrast, s-block metal ions lack suitable orbitals and form far fewer stable complexes.
Negatively charged ligands are attracted more strongly to positively charged metal ions.
This stronger electrostatic attraction often leads to more stable complexes compared with those formed using neutral ligands.
However, stability also depends on ligand size and the metal ion’s charge and radius.
Yes, some ligands have more than one lone pair and can form multiple coordinate bonds with the same metal ion.
These are known as multidentate ligands.
Such ligands increase complex stability because several coordinate bonds must be broken simultaneously for the ligand to detach.
Small ligands such as water and ammonia can pack closely around the metal ion, allowing six coordinate bonds to form.
This arrangement minimises electron pair repulsion while maximising metal–ligand interactions.
Tetrahedral complexes are more likely when ligands are larger or when the metal ion has lower charge density.
Square planar geometry arises from specific electronic configurations that favour planar ligand arrangements.
These electronic factors reduce repulsion within the metal’s d orbitals when ligands lie in one plane.
As a result, only certain transition metal ions form stable square planar complexes rather than tetrahedral ones.
Practice Questions
Define the terms ligand and coordination number as used in transition metal chemistry.
(2 marks)
Ligand defined as an ion or molecule that donates a lone pair of electrons to a central metal ion (1 mark).
Coordination number defined as the number of coordinate bonds between a central metal ion and surrounding ligands (1 mark).
A transition metal ion forms a complex with four ligands.
a) Define the term complex ion.
b) State two different geometrical shapes that a coordination number 4 complex can adopt.
c) Explain why ligands are able to form coordinate bonds with transition metal ions.
(5 marks)
a) Complex ion definition (1 mark)
A charged species consisting of a central metal ion bonded to one or more ligands by coordinate bonds.
b) Shapes of coordination number 4 complexes (2 marks)
Tetrahedral (1 mark).
Square planar (1 mark).
c) Explanation of coordinate bond formation (2 marks)
Ligands possess lone pairs of electrons (1 mark).
The lone pair is donated to an empty orbital on the metal ion to form a coordinate (dative covalent) bond (1 mark).

